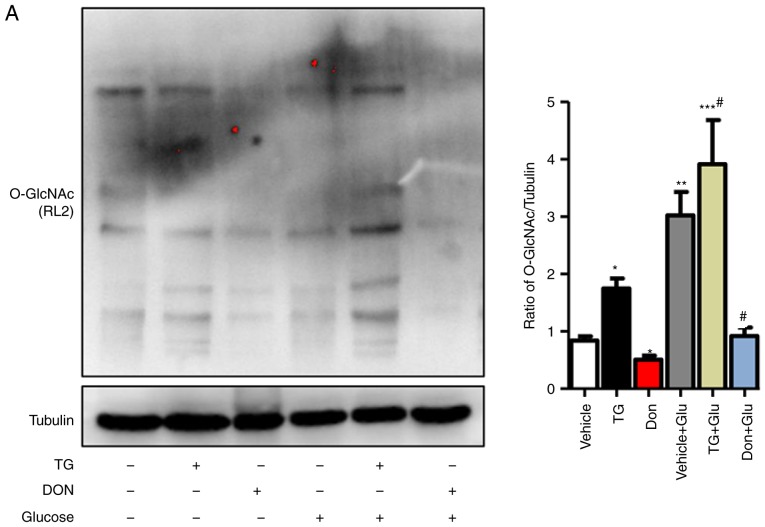
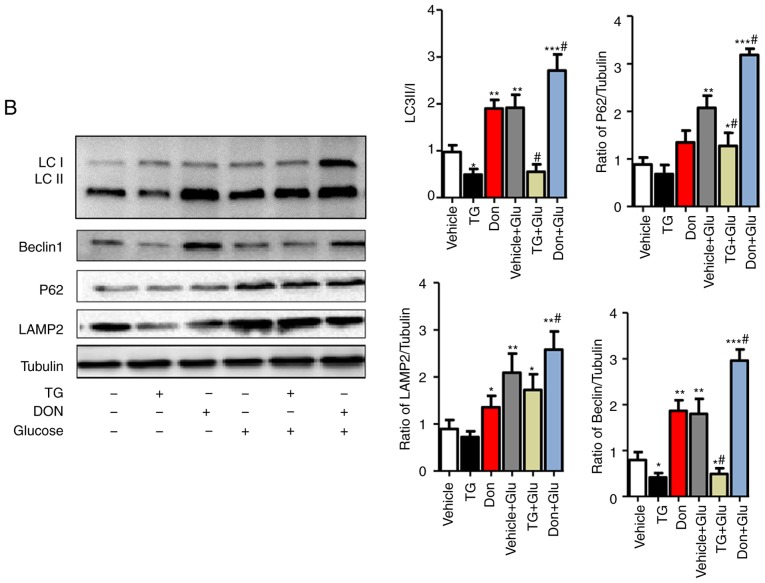
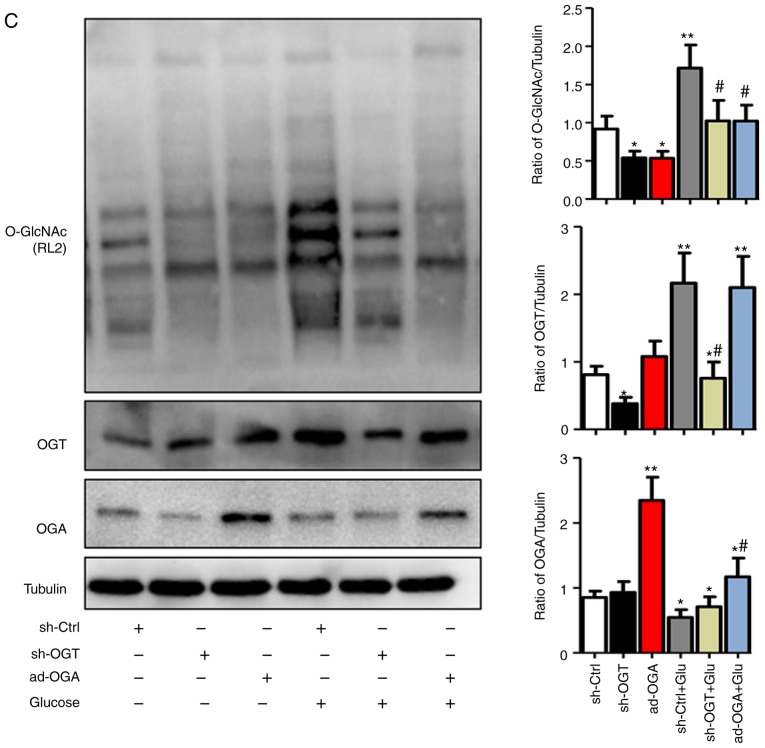
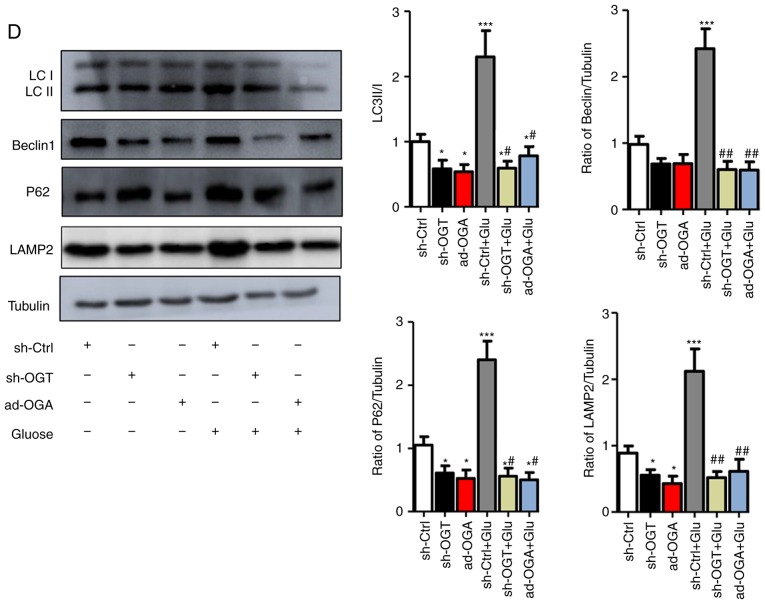
Figure 3.
Increased O-GlcNAc modification inhibits autophagic flux in NRCMs under high-glucose conditions. NRCMs were exposed to high glucose (25 mM) and were treated with TG (5 µM) or Don (40 µM) for 24 h. (A) Supernatants were extracted, and the expression of O-GlcNAc (RL2) was analyzed by western blot analysis. (B) Expression levels of LAMP2, Beclin-1, P62, and LC3II/I in each group were detected by western blot analysis. NRCMs were transfected with sh-OGT or ad-OGA for 48 h and were exposed to high glucose (25 mM, Glu group) for 24 h. (C) Supernatants were extracted, and the expression levels of O-GlcNAc (RL2), OGT, and OGA were analyzed by western blot analysis. (D) Expression levels of LAMP2, Beclin-1, P62, and LC3II/I in each group were detected by western blot analysis. Data are expressed as the mean ± standard error of the mean (n≥6). *P<0.05, **P<0.01 and ***P<0.001, vs. Vehicle group; #P<0.05 and ##P<0.01, vs. Glu group. Tubulin was the loading control. NRCMs, neonatal rat cardiomyocytes; O-GlcNAc, O-linked β-N-acetylglucosamine; STZ, streptozotocin; sh-OGT, OGT-knockdown adenovirus; ad-OGA, OGA-overexpression adenovirus; Ctrl, control; Glu, glucose; Don, 6-diazo-5-oxo-L-norleucine; TG, thiamet G; OGT, O-GlcNAc transferase; OGA, O-GlcNAcase; LAMP2, lysosome-associated membrane protein 2; LC3, microtubule-associated protein 1 light chain 3α.