Fig. 2. A PIPRSP motif in LAT promotes the phosphorylation of LAT.
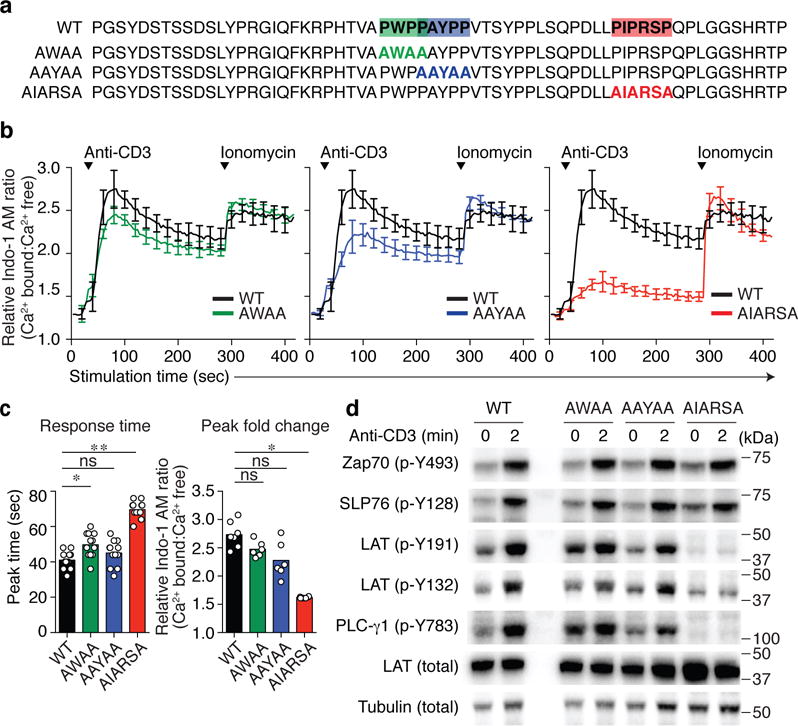
(a) Location of three proline-rich motifs, PWPP, PAYPP and PIPRSP, highlighted in green, blue and red respectively. Proline to alanine mutations in each of the motifs are shown below the wild type sequences (WT) of human LAT. (b) CRISPR/Cas9 generated LAT-deficient J.LAT cells were reconstituted with wild type LAT (WT), or each proline-rich motif mutant LAT (AWAA, AAYAA, or AIARSA). Cells were loaded with Indo-1 AM, stimulated with 0.5 μg/ml of anti-CD3 and the changes in relative calcium-sensitive fluorescence ratios over time for 400 sec are shown in b (mean ± s.d.; n = 6 technical replicates). Data are representative of five independent experiments with similar results. (c) Bar graph (left) shows the analysis of response time to reach the peak (mean ± s.d.; n = 12 technical replicates in five independent experiments). Experiments have been done at least five times with similar results. *P = 0.0029, **P < 0.0001 (exact value). ns, not significant; two-tailed Mann-Whitney test. Bar graph (right) shows the quantification of the Indo-I AM ratio fold change in peak (mean ± s.d.; n = 7 technical replicates in two experiments for WT, n = 6 technical replicates in two experiments for AWAA, AAYAA, AIARSA). Data are representative for at least five independent experiments. *P = 0.0012, ns, not significant; two-tailed Mann-Whitney test. (d). J.LAT cells reconstituted with WT, or mutant AWAA, AAYAA, AIARSA LAT were left unstimulated or treated with 0.5 μg/ml anti-CD3 for 2 min. Immunoblot analyses are shown. Data are representative of three experiments. Numbers to the right of cropped blots indicate mobilities of molecular mass marker proteins (kDa).
