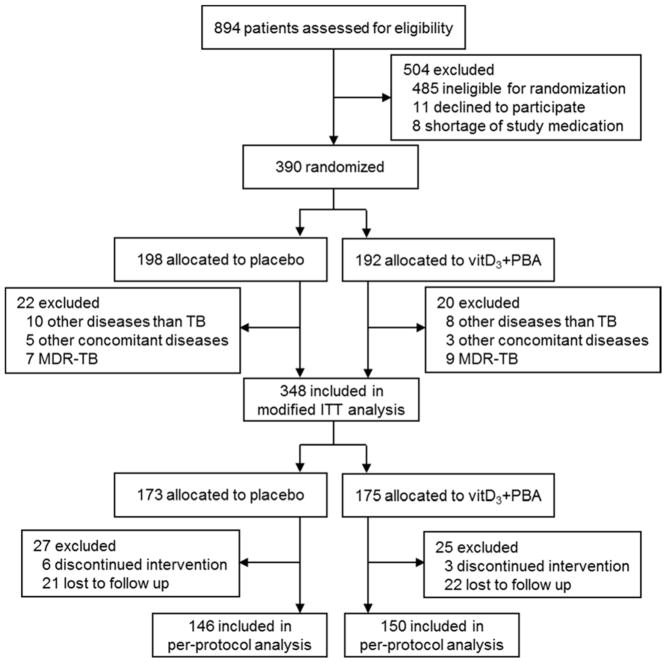
Fig. 1.
Trial profile. Consort flow diagram of patients with suspected pulmonary TB, from screening to analysis. Patients ineligible for randomization included HIV infection (n=418), relocation after diagnosis (n=17), age <18 years (n=16), non-TB pleural effusions (n=16), TB relapse (n=9), too weak/old (n=4), >5 days into TB chemotherapy (n=2), pregnancy (n=2) and mental health problems (n=1). Diseases other than TB included pulmonary fibrosis (n=14), cancer (n=2), and pulmonary thromboembolism (n=2) while other concomitant diseases included HIV infection (n=2), liver disease (n=3) and renal disease (n=3). Discontinued intervention included patients with liver toxicity (n=3), adherence failure (n=5) and cancer (n=1). Lost to follow up included patients who withdraw their consent (n=30), moved from study area (n=12), or were imprisoned (n=1). Patients who dropped out from the placebo treatment at week 0: n=11, week 4: n=9, week 8: n=5 and week 16: n=2. Patients who dropped out the vitD3+PBA treatment at week 0: n=14, week 4: n=5, week 8: n=5 and week 16: n=1.