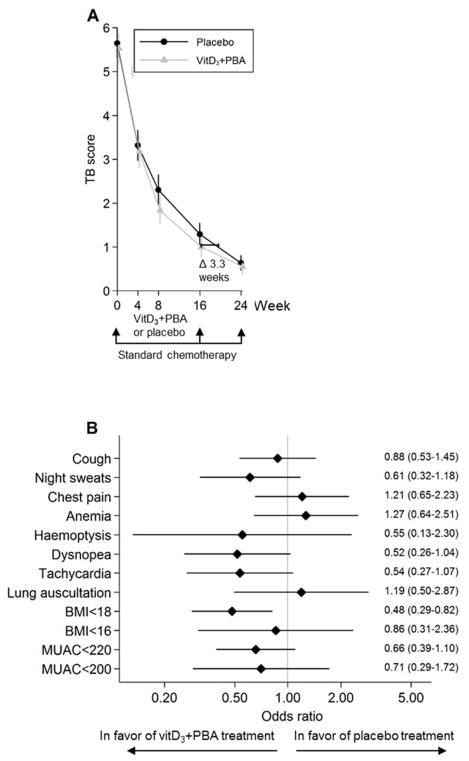
Fig. 2.
Primary efficacy analyses. (a) The primary clinical TB score was assessed at baseline and at weeks 4, 8, 16, and 24 after initiation of anti-TB chemotherapy. Adjunct vitD3+PBA treatment was provided during the first 16 weeks of standard care. The efficacy analysis included comparison of the vitD3+PBA and placebo treatment between week 0 and week 8. Crude data from the mITT cohort are presented as the mean and 95% CI. The blue line (circles) represents placebo while the red line (triangles) represents vitD3+PBA treatment. The horizontal bar indicate the estimated difference (given a linear reduction of the TB score) in weeks that it would take to reduce the primary TB score in the placebo group to a level comparable to the TB score in the vitD3+PBA group assessed at the end of adjunct treatment at week 16. (b) Forrest plot showing the odds ratio of the individual diseases symptoms included in the primary efficacy analysis. The estimate and 95% CI at week 8 are shown.