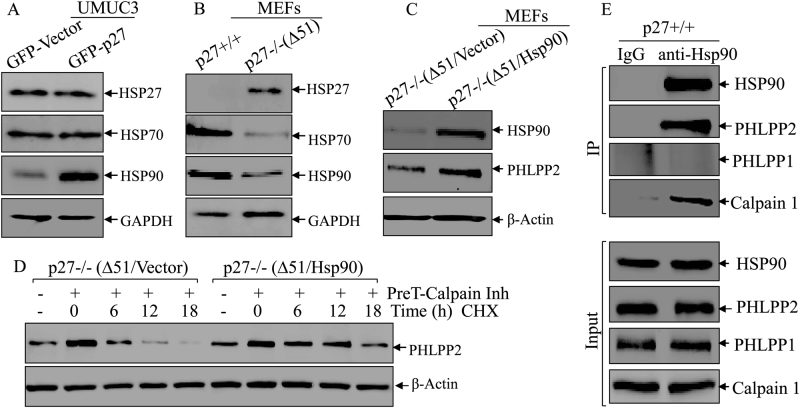
Fig. 3.
Hsp90 interacted with PHLPP2 and prevented it from degradation. a, b Western blot was used to detect the protein level of Hsp27, Hsp70, and Hsp90 in UMUC3(Vector) vs UMUC3(GFP-p27) cells (a), p27+/+ vs p27−/−(Δ51) cells (b). GAPDH was used as protein loading control. c Western blot was used to identify the protein level of Hsp90 in p27−/−(Δ51) cells stably transfected with ectopic Hsp90. PHLPP2 protein was evaluated in the indicated cells, β-Actin was used as protein loading control. d p27−/−(Δ51) and p27−/−(Δ51/Hsp90) cells were pre-treated with Calpain inhibitor for 12 h and the cells were then subjected to protein degradation assay in the presence of CHX and absence of Calpain inhibitor for the indicated time periods. The cell extracts were subjected to western blot to analyze PHLPP2 protein degradation rates among the indicated cells. β-Actin was used as protein loading control. e IP assay, described in “Materials and methods”, was applied to see the direct binding of Hsp90 and its substrates. We used anti-Hsp90 antibody to pulldown the proteins that could bind to Hsp90, and WB was used to detect the protein abundance of PHLPP2, PHLPP1 and Calpain1