Abstract
Introduction
High failure and recurrent prolapse remains an important issue for pelvic organ prolapse (POP) surgery. The posterior intravaginal slingplasty (PIVS) is a minimally invasive, transperineal technique providing level I support, by creating neo-sacrouterine ligaments using a mesh. In order to reduce the POP recurrence rate, achieve a safer apical support and thereby better functional outcomes, we attached PIVS tape to the sacrospinous ligament bilaterally and compared the anatomical and functional outcomes for our modified technique versus the original PIVS.
Material and methods
We evaluated 368 patients, with a symptomatic pelvic organ prolapse in various grades, who had undergone a total pelvic floor reconstruction. Seventy-seven of 368 (21%) patients underwent the original PIVS, 291 (79%) patients were treated by the modified PIVS. When necessary, the following procedures were added: anterior transobturator mesh, posterior wall repair, perineal body repair and suburethral transobturator sling. All had follow-up checks for at least one year. The primary outcome was an objective cure, defined as grade 0 or grade 1 according to Baden-Walker. Secondary outcomes were prolapse recurrence, symptoms, visual analogue scales for satisfaction, quality of life, recommendation, reoperation rates and presence of complications.
Results
The total reconstructions we made, using each technique, were successful. We achieved an apical success rate of 97 to 96%, on average, with the modified and original IVS respectively. We found a statistically significant improvement in urge incontinence and frequency symptoms than the original PIVS with our modified technique.
Conclusions
Modified PIVS in combination with concomitant procedures generates high anatomical and functional cure rates with low complications and recurrences.
Keywords: integral theory, posterior intravaginal slingplasty, rectocele, cystocele, sacrospinous ligament fixation
INTRODUCTION
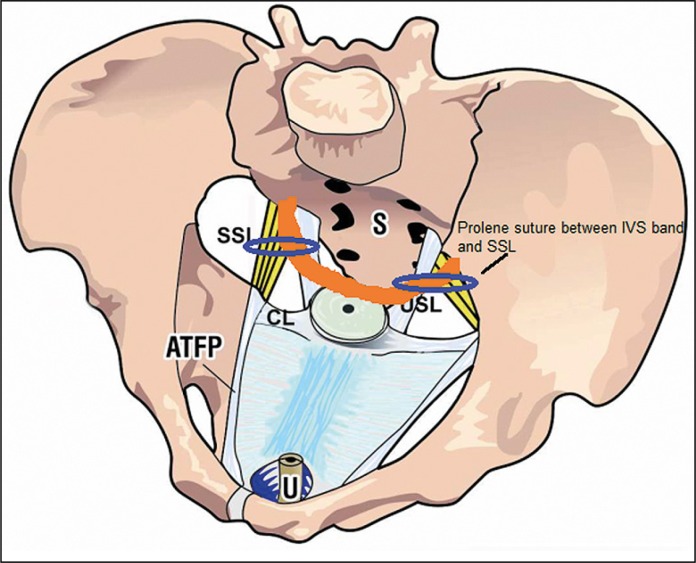
Although pelvic organ prolapse and pelvic floor dysfunction are multifactorial and in some cases are caused by neuromuscular deficits, deficient connective tissue plays an important role. First, DeLancey showed the significance of connective tissue structures for organ suspension and proposed three levels of vaginal support [1]. Then, Petros et al. created a new vaginal strategy for the pelvic floor surgery (now known as the Integral System) based on the Integral Theory which regards both, symptoms and organ prolapse as being caused by lax suspensory ligaments [2] and developed a new surgical principle: creation of an artificial collagenous neo-ligament to repair the loose ligaments. This was first applied for reinforcing the pubourethral ligament in order to cure stress urinary incontinence (SUI), today known as tension-free vaginal tape (TVT) and secondly PIVS operation, for renewing the uterosacral ligaments in order to cure an uterine/apical prolapse (level 1) [3]. Amreich firstly described the sacrospinous ligament fixation (SSLF) for cases also with the uterine/apical prolapse. We started with the original PIVS operation for pelvic organ prolapse (POP) surgery in 2008. Despite successful results, there were poor outcomes in some small studies [4, 5] and in our own experience [6], therefore this procedure was not sufficient enough to bring the apex far back. Considering this, from October 2010 we continued the operations with the Goeschen's modified PIVS technique [7] to provide better apical support and thereby better functional outcomes by combining the two techniques used for level I repair (Figure 1, Figure 2B). In our opinion, attaching the sacrospinal sutures to the vaginal mucosa or approximating the damaged tissue to damaged tissue, not reinforced by artificial mesh, reduced success. Therefore, we have thought to connect the sacrospinal sutures to the IVS band.
Figure 1.
Level I view from above: SSL – sacrospinous ligament; S – sacrum; CL – cardinal ligament; USL– uterosacral ligament; ATFP – arcus tendineus fasciae pelvis; orange – the IVS tape.
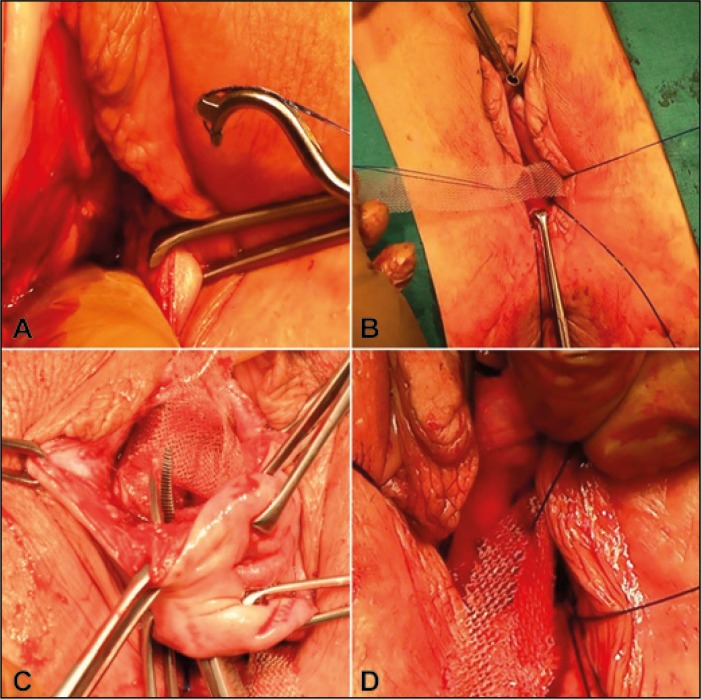
Figure 2.
A. After transverse 4 cm long incision 1,5 cm below the cervix or hysterectomy scar, the sacrospinous ligaments are freed from adherent tissue by digital blunt dissection. Insertion of two 2-0 prolene sutures through the ligament using a special designed sacrofix instrument on both sides. B. One of the two prolene sutures is brought through the middle of the tape leaving a distance of 4 cm for the contralateral prolene suture. C. A channel around the cervix from the posterior to the anterior incision is created with an overholt forceps on both sides, the posterior two arms are placed around the cervix subepithelially and and pulled back into the posterior incision area. D. The two remaining sacrospinous prolene sutures are connected with the posterior arms of the mesh close to the cervical ring on each side.
The vaginal approach also constitutes the physiological vaginal axis and allows level III repair for a stable and narrow hiatus genitalis to prevent the recurrence using the same route (Figure 3). Because of the poor tissue we always use mesh support, in order to reinforce the pubocervical fascia (PCF) and reconnection of the detached vaginal wall to arcus tendineus fasciae pelvis (ATFP), if anterior repair was necessary. Lloyd et al. showed that the vaginal length differs from 6.5–12.5 cm [8]. That means if the mesh is too small it will cause dyspareunia, bladder and rectal problems due to a short vagina. If the mesh is too large, it wrinkles, creates erosions and generates the same problems. Industrial pre-fabricated meshes can explain the high, up to 17%, mesh erosion rate in the literature [9]. We performed posterior repair according to the tent theory, especially in patients with difficulty in defecation and with different degrees of posterior wall defect [10]. That means, ”If the top of a tent caves in, the walls may follow. Secure the top first.” Our vaginal procedure combines the principles of Integral Theory based on DeLancey's suggestions and traditional proven surgery. The purpose of this study is to compare the long-term anatomical and functional outcomes of the original PIVS method (n: 77) with the modified technique (n: 291).
Figure 3.
Normal physiological vaginal axis appears at the end of procedure.
MATERIAL AND METHODS
This was a retrospective study based on 368 patients, who had symptomatic POP of some degree and symptoms such as frequency, nocturia, urgency with or without incontinence, chronic pelvic pain, stress incontinence, stool outlet difficulty during August 2008 and September 2015. Patients who were not suffering from prolapse symptoms, such as a vaginal bulge, but upon examination had a loss of pelvic support with urinary and defecation problems, resistant to conservative and medical treatment, were also included. Seventy-seven of 368 (21%) patients underwent the original PIVS until October 2010, after that 291 (79%) patients were treated by the modified PIVS. Two hundred sixty-seven of the patients in the modified PIVS group are patients whose data we have previously published. According to the patients’ complaints and the pre-op evaluation, the following procedures were added: anterior transobturator mesh, posterior wall repair, perineal body repair and suburethral transobturator sling. The grade of the prolapse was assessed using the Halfway Classification System and according to Baden-Walker between grades 1 and 4. All patients presented a clinically evident fornix prolapse. Distribution of prolapse grades: in the modified PIVS group, grade 2 or more apical prolapse rate was 91%; while the same ratio was around 60% in the original PIVS group. Grade 4 prolapse rates were 61% and 4% respectively.
For the evaluation of SUI, a stress test was performed. Patients with grade 3–4 prolapse were evaluated after replacement of the prolapse with a speculum. All patients completed a questionnaire indicating age, body mass index (BMI), menopause status, parity, systemic diseases, medications, past gynecologic and uro-gynecologic history, previous operations, urinary symptoms with a 24-hour urinary diary, defecation symptoms and pelvic pain (Tables 1, 2). In all the patients, symptoms derived from the questionnaire were ticked off in a diagnostic algorithm, indicating the zone of connective tissue damage as anterior, middle and posterior [11]. Preoperative and postoperative data were reviewed retrospectively from the patient files, which had been prospectively recorded for each patient.
Table 1.
Patient demographics
| Modified PIVS |
Original PIVS |
p | |
|---|---|---|---|
| n = 291 mean ±SD (range) n (%) |
n = 77 mean ±SD (range) n (%) |
||
| Age (years) | 54 ±11 (28–81) | 51 ±11 (27–84) | <0.05 |
| Parity | 3.6 ±1.7 | 3.4 ±1.9 | 0.59 |
| Body mass index (kg/m2) | 28.1 ±4.4 | 28.1 ±5.3 | 0.94 |
| Patients with menopause | 181 (62%) | 42 (55%) | 0.22 |
| Menopausal duration | 12.4 ±7.6 | 10.5 ±7.4 | 0.14 |
| Previous hysterectomy | 30 (10.3%) | 8 (10.4%) | 1.00 |
| Previous pelvic organ prolapse surgery | 18 (6.2%) | 4 (5.2%) | 1.00 |
| Previous anti-incontinence surgery | 6 (2.1%) | 5 (6.5%) | 0.06 |
| Hospitalization (days) | 2.8 ±1.2 | 3.2 ±0.9 | <0.05 |
| Follow-up (months) | 27.5 ±15.4 (12–65) | 39.8 ±19.8 (12–68) |
Table 2.
Anatomic and functional results at least one year after surgery
| Modified PIVS n = 291 (79%) | p | Original PIVS n = 77 (21%) | p | p | ||
|---|---|---|---|---|---|---|
| Anterior wall | Gr. 2–4 cure | 165/190 (87%) | <0.001 | 59/63 (94%) | <0.001 | 0.17 |
| Posterior wall | Gr. 2–4 cure | 268/277 (97%) | <0.001 | 46/49 (94%) | <0.001 | 0.39 |
| Apex | Gr. 2–4 cure | 257/264 (97%) | <0.001 | 44/46 (96%) | <0.001 | 0.62 |
| Stress incontinence | Cure De novo | 202/212 (95.3%) 5/79 (3%) | <0.001 | 60/64 (93.7%) 2/13 (13%) | <0.001 | 1.00 0.3 |
| Urge incontinence | Cure De novo | 85/94 (90%) 10/197 (5%) | <0.001 | 26/35 (74%) 3/42 (7%) | <0.001 | <0.05 0.70 |
| Pelvic pain | Cure De novo | 88/91 (97%) 0/200 (0%) | <0.001 | 20/21 (95%) 0/56 (0%) | <0.001 | 0.56 1.00 |
| Urgency | Cure De novo | 103/116 (89%) 9/175 (5%) | <0.001 | 38/50 (76%) 2/27 (7%) | <0.001 | 0.06 0.64 |
| Nocturia | Cure De novo | 36/57 (63%) 16/234 (7%) | <0.01 | 13/21 (62%) 8/56 (14%) | 0.38 | 1.00 0.10 |
| Frequency | Cure De novo | 106/120 (88%) 7/171 (4%) | <0.001 | 31/44 (71%) 5/33 (15%) | <0.001 | <0.01 <0.05 |
| Stool outlet difficulties | Cure De novo | 50/62 (81%) 6/229 (3%) | <0.001 | 11/11 (100%) 3/66 (5%) | 0.06 | 0.19 0.42 |
| Patient’s satisfaction VAS (Visual Analogue Scale) | 9.4 ±1.7 | 8.5 ±2.6 | <0.001 | |||
| 8–10 | 263 (90%) | 60 (78%) | ||||
| 4–7 | 21 (7.2%) | 11 (14%) | ||||
| 1–3 | 7 (2.4%) | 6 (7.8%) | ||||
| Recommend | 281 (97%) | 70 (91%) | 0.06 | |||
| Re-operation (pelvic organ prolapse surgery) | 13 (4.5%) | 7 (9.1%) | 0.15 | |||
| Re-operation (anti-incontinence surgery) | 9 (3.1%) | 2 (2.6%) | 1.00 | |||
| Quality of life | ≥12 m. | ≥12 m. | ||||
| Much better | 233/291(80%) | 53/77 (69%) | <0.49 | |||
| A little better | 26/291(8.9%) | 9/77(11.6%) | <0.52 | |||
| About the same | 23/291 (7.9%) | 9/77(11.6%) | <0.37 | |||
| A little or much worse | 9/291 (3%) | 6/77 (7.8%) | <0.10 |
Frequency has been defined as either eight or more micturitions per day (with day being defined as during the daytime), urgency as a sudden compelling desire to pass urine, nocturia refers to waking at night two or more times to void. Stress incontinence is a complaint of involuntary loss of urine on effort or physical exertion or on sneezing or coughing, urgency is a urinary incontinence complaint of involuntary loss of urine associated with urgency (at a threshold of 2 per day) [12]. Stool outlet difficulties are characterized as a feeling of incomplete emptying, straining at defecation, pain during evacuation, assisted digital evacuation. Pelvic pain was defined as a low dragging abdominal pain or deep sacral backache that is not associated with symptoms suggestive of lower urinary tract, sexual, bowel, or gynecological dysfunction [13]. Bladder emptying difficulties include the complaints that the bladder does not feel empty after micturition, slow urine stream, starting and stopping or dribbling.
The criteria for the symptomatic improvement postoperatively are detailed as nocturia – less than two times per night; urge incontinence – no wet episodes per day; frequency – less than eight episodes per day; stress incontinence and a negative cough test. Defecation difficulties – normal defecation. Pelvic pain – self-assessed 80% improvement or cure over the baseline symptom at the ≥12 month visit; urgency – no desire to pass urine and bladder emptying difficulties – no symptoms.
Surgical technique in detail
Level I repair was achieved by inserting a polypropylene tape in the exact position as the uterosacral ligaments. Seventy-seven patients had a PIVS as originally described [3] for repair of level I. In addition to an original PIVS as explained previously [7], in 291 patients the tape was attached to the sacrospinous ligament on both sides with a prolene suture, using a sacrofix device, according to Goeschen (Handke Medizintechnik Gmbh Germany) (Figure 2 A,B). If necessary the other levels (II and III) were repaired as well. In cases of a concomitant anterior wall prolapse a 4-arm-ATOM (anterior transobturator mesh) was passed, using the traditional transobturator technique in the original PIVS group. In order to restore the cardinal ligaments, in the modified PIVS group (n: 291) the posterior arms of 4-arm mesh were placed around the cervix or cuff line by pulling them back into the incision area with the ‘overholt’ forceps. These two arms were attached to the remaining sacrospinous sutures bilaterally, as well (Figure 2 C,D). Rectovaginal fascia (level II) and a widened genital hiatus (level III) were restored using a posterior ‘bridge’ and the approximation of the perineal body by horizontal mattress sutures. Patients with SUI received a suburethral transobturator sling (preoperatively and perioperatively diagnosed patients). Full thickness of the mucosa was cut and closed in two layers. Concomitant operations are detailed in Table 4. The first and third authors performed the surgeries and examinations. In all procedures, self-tailored polypropylene monofilament meshes (Atrium®) were used. All menopause patients were treated with local estrogen pre- and postoperatively. During the operations, a single dose of ceftriaxone (2 g) was applied and thrombosis prophylaxis was administered.
Table 4.
Operation frequency
| Modified PIVS (n = 291) | Original PIVS (n = 77) | |
|---|---|---|
| Hysterectomy | – | – |
| Posterior bridge repair | 256 (88%) | 42 (54.5%) |
| Anterior transobturator mesh | 177 (60.9%) | 61 (79.2%) |
| PIVS | 291 (100%) | 77 (100%) |
| Bilateral sacrospinous ligament fixation | 291 (100%) | – |
| Transobturator tape | 212 (72.9%) | 64 (83.1%) |
Postoperative follow-up visits were performed after three months, one year and yearly thereafter. Patients with at least one year and complete follow-up data were included in this study. The primary outcome was an objective cure, defined as grade 0 or grade 1 according to Baden-Walker. Subjective cure was defined as the absence of a vaginal bulge, according to questions two and three from the Pelvic Floor Distress Inventory (PFDI). Secondary outcomes were prolapse recurrence, symptoms, recommendation, reoperation rates and presence of complications. Visual analogue scale (VAS) determined the subjective satisfaction of patients’ with their surgery (from 1 to 10; 1 represented completely dissatisfied, 10 – very satisfied). How the operation affected their quality of life was questioned with the Patient Global Impression of Change (PGIC) as “little or much worse”, “about the same”, a “little better” and “much better” [14].
The Ethics Committees of the Pamukkale University approved this study in 2017. The analysis was performed using the Student's T-test, Mann-Whitney U and the chi-square test (exact Fischer Test or paired data by McNemar's test). A p-value less than ≤0.05 was considered to be statistically significant.
RESULTS
Table 1 presents the demographic characteristics of patients. There were statistically significant differences between groups in only the age and hospitalization days. Although most of the patients were in their 50s, the modified PIVS group was statistically older (the mean age in both groups was 51 to 54 years) and had a shorter stay at the hospital (the mean days 2.8 to 3.2). Anatomical and functional results are shown in Table 2. Both groups showed significant improvements of the apical prolapse: 97% (modified) and 96% (original) compared with the preoperative status but there was no statistical significance between the two groups in the follow-up of mean 27.5 and 39.8 months. However, in grade 3–4 patients, the modified PIVS lead to higher (95%) success rates than the original PIVS (88%), although statistically they were not significant. Patients, who underwent paraurethral midurethral sling for SUI, had the same success rate in both groups (96% vs. 97%). The two techniques created a significant improvement of symptoms in both groups (Table 2). The comparison of the functional outcome is as follows, the modified group showed a significantly better improvement than the original PIVS group in urge incontinence (90% vs. 74%) and in frequency (88% vs. 71%). The cure rate of urgency was also better in the modified PIVS group (89% vs. 76%), reaching no significant difference. There was no significant difference in results for nocturia, stool outlet difficulties and pelvic pain. Whereas the de novo nocturia rate was greater in the original PIVS group than in the modified PIVS group (14% vs. 7%), but with no statistical difference. In both groups, the patients' (quality of life (QoL) significantly improved after surgery. Most women reported that they were either “much better” (80%) or “a little better” (8.9%) on the PGIC in the modified groups and 69% and 11.6% in original PIVS group, respectively. Still the severely affected patients ≥1 year after surgery, were statistically more frequent in the original PIVS group than in the modified PIVS group: 7.8% vs. 3%. Both groups highly recommended the surgery to others; modified PIVS group 97% vs. the traditional PIVS group 91%, however the difference was not statistically significant. For at least 12 months, the average VAS for satisfaction with surgery was 9.4 ±1.7 for the modified group and 8.5 ±2.6 for original PIVS group. In the follow-up, 97 % of patients in the modified PIVS group and 94.7% of patients in the original PIVS responded ‘no’ to PFDI questions two and three for vaginal bulge symptoms.
Intraoperative and postoperative complications are summarized in Table 3. Only one patient required a postoperative blood transfusion, and another presented with vaginal bleeding 10 days after surgery, which was treated with sutures. One extraperitoneal haematoma in the cranial rectovaginal space was resorbed spontaneously. A total of eight patients had only small local mesh erosion. In these patients, the protruded mesh was removed and covered by vaginal skin. Other complications such as embolic problems and pyrexia did not occur.
Table 3.
Intraoperative, early postoperative and postoperative complications
| Intraoperative & early postoperative n (%) |
Postoperative ≥1 year n (%) |
||||
|---|---|---|---|---|---|
| Modified PIVS | Original PIVS | Modified PIVS | Original PIVS | ||
| Bladder injury | 8 (2.7%) | 2 (2.6%) | |||
| Rectal injury | 3 (1%) | – | |||
| Blood transfusion | 1 (0.3%) | – | |||
| Hematoma | 1 (0.3%) | – | |||
| Wound infection | 1 (0.3%) | – | |||
| Mesh erosion | 5 (1.7%) | 3 (3.9%) | |||
| Re-operation (pelvic organ prolapse surgery) | 14 (4.8%) | 6 (7.8%) | |||
| Re-operation (anti-incontinence surgery) | 9 (3%) | 2 (2.6%) | |||
DISCUSSION
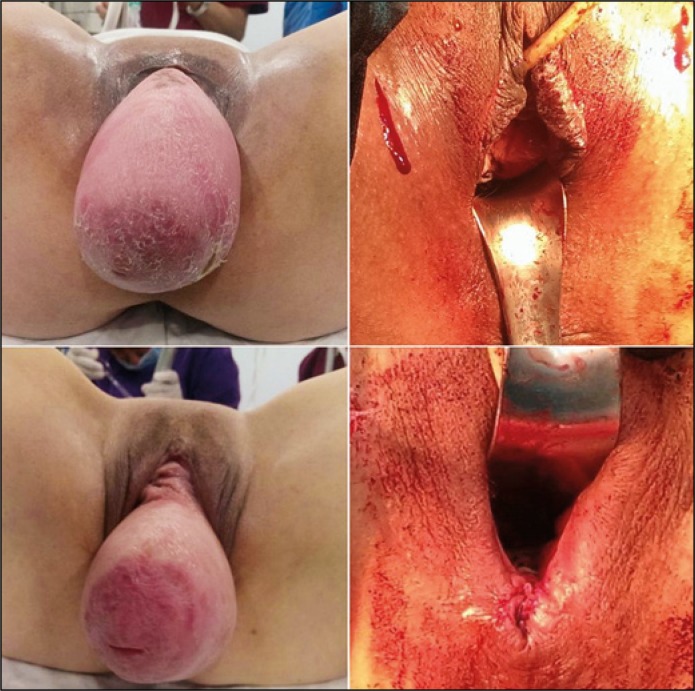
Because of high failure, reoperation rates, and low patient satisfaction in the past, surgeons have been searching for the ideal technique for POP surgery. Lawndy et al. reported de novo symptoms in 63%, POP recurrence in 34% and surgical complications in 29% as the most important fears of patients [15]. Many authors have pointed to the importance of the apex for adequate POP repair [16, 17]. For us this means “secure the apex first”. Although there is a small series of poor results [4, 5], the PIVS technique is well known and achieved a favorable anatomical success ranging from 83% to 100% [18]. Tseng et al. reported, in a systemic review, an overall cure rate of 84.6% (range 69–100%) for SSLF [19]. By combining these two techniques in the same patient, we thought that we could achieve a more permanent and better anatomical success and thus functional outcomes. We achieved an apical success rate of 96% to 97% on average with the modified and original PIVS respectively. Although not statistically significant, in patients with grade 3–4 prolapse, the cure rate was more in favor of the modified group (95% to 88%). Therefore, we safely recommend it, especially in grade 4 prolapse (Figure 4).
Figure 4.
Preop and postop images of grade 4 prolapse (enteroptosis: complete protrusion of all pelvic organs as well as intestine and mesenterium).
Another important evaluation measure of the POP surgery is the reoperation rate due to recurrence. Denman et al. measured a ten-year reoperation rate of 17% [20]. In the modified group our reoperation rate was 4.5% and in the original PIVS group it was 9.1%. In recent years, there have been increasing numbers of concerns about the use of mesh. Australia recently banned transvaginal mesh products for the treatment of pelvic organ prolapse. Our mesh erosion rates were 1.7% after the modified PIVS vs. 3.9% after the original PIVS. The reason for this low frequency was, in our opinion, the full-thickness dissection, the use of pre-postop estrogen, a two-layer closure of the mucosa, reducing the amount of mesh by not using mesh in the posterior compartment and not using an industrial prefabricate, but self-tailored mesh appropriate for the defect.
Although the most uncomfortable symptoms for the patients seen were vaginal bulge (53.2%) and incontinence (39.9%), in our series many additional functional complaints came into view as the anamnesis deepened, such as symptomatic overactive bladder (OAB) described as urgency, nocturia and frequency, and difficulty in defecation, bladder emptying problems, and pelvic pain. Liang et al. assessed urinary symptoms during patient interviews and reported frequency in 73.4%, nocturia in 42.8%, urgency in 47%, urgency incontinence in 26%, stress incontinence in 46.1%, and voiding difficulty in 38% [21]. The curable effect of the suspensory ligament repair on OAB symptoms has been shown in a prospective, urodynamically controlled observational study [3]. Meanwhile, there are many publications about the positive effects of POP surgery on OAB [22, 23]. On the other hand, Zahravi et al. still support the general view of International Continence Society (ICS) and they believe that patients with OAB symptoms tend to have a smaller bladder capacity and a higher incidence of detrusor overactivity [24].
Regarding the functional symptoms, the difference before and after treatment was statistically highly significant in both groups. This also proves the logic of the Integral Theory. The posterior IVS tape is not only the renewal of the uterosacral ligament (USL), but also a re-attachment of the uterus or vagina to the levator plate, in order to allow the backward force to open and close the bladder and rectum. Similarly, pubourethral ligament (PUL) laxity is the principal cause of stress incontinence and can also be an important cause for urgency. Boer et al. reported, in accordance with our modified PIVS group results, de novo OAB symptoms in 5–6% of women after surgery [25]. Our de novo rates for all symptoms were lower in the modified PIVS group (4–7.9%) than in the original PIVS group (7.1–15%), but reached statistical significance only for frequency (4% vs. 15%). Complete restoration of all deficient connective tissue simultaneously, a vaginal approach, uterine preservation, physiological vaginal axis, and no use of a ready-kit are the strengths of our work. Its retrospective nature is the weakness of our research. The difference in the follow-up time between the two groups and the start of the modified PIVS group later can be interpreted as experience against the original PIVS group.
CONCLUSIONS
Our data shows an improvement in bladder function as well as prolapse cure with each procedure. Our modified PIVS technique has created a better success for urge incontinence and frequency. Additionally, we also found the tendency of the combined approach to be more reliable and generate better results, especially in grade 4 prolapse. After a relatively short follow-up period, the additional fixation of the PIVS tape to the sacrospinous ligament brought better functional results. Due to the fact that this research is still in its infancy, the long-term result remains to be seen and whether this technique will continue.
Acknowledgments
We acknowledge and thank Prof. Petros, who with his colleague Prof. Dr. Ulmsten developed the Integral Theory and related diagnosis and treatment methods, which help us and especially our patients. We would also like to thank Ali Ersin Zumrutbas, MD for his advice and recommendations concerning the article and for his help in the first publication, which is the basis for this study.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717–1724. doi: 10.1016/0002-9378(92)91562-o. [DOI] [PubMed] [Google Scholar]
- 2.Petros PE, Ulmsten U. An integral theory and its method for the diagnosis and management of female urinary incontinence. Scand Urol Nephrol Supl. 1993;153:1–93. [PubMed] [Google Scholar]
- 3.Petros PE. New ambulatory surgical methods using an anatomical classification of urinary dysfunction improve stress, urge, and abnormal emptying. Int J Urogynecol. 1997;8:270–278. doi: 10.1007/BF02765483. [DOI] [PubMed] [Google Scholar]
- 4.Mattox TF, Moore S, Stanford EJ, Mills BB. Posterior vaginal sling experience in elderly patients yields poor results. Am J Obstet Gynecol. 2006;194:1462–1466. doi: 10.1016/j.ajog.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 5.Hinoul P, Vanspauwen R, Smajda S, Roovers J-P. The Posterior Intravaginal Slingplasty treatment for apical prolapse: 3 years experience in a single centre setting. Facts Views Vis Obgyn. 2010;2:1–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Goeschen K, Gent H-J. Das posteriore Fornixsyndrom. Frauenarzt. 2004;45:104–112. [Google Scholar]
- 7.Caliskan A, Goeschen K, Zumrutbas AE. Long term results of modified posterior intravaginal slingplasty (P-IVS) in patients with pelvic organ prolapse. Pelviperineology. 2015;34:94–100. [Google Scholar]
- 8.Lloyd J, Crouch NS, Minto CL, Liao LM, Creighton SM. Female genital appearance: ‘normality’ unfolds. BJOG. 2005;112:643–646. doi: 10.1111/j.1471-0528.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 9.Min H, Li H, Bingshu L, et al. Meta- analysis of the efficacy and safety of the application of adjuvant material in the repair of anterior vaginal wall prolapsed. Arch Gynecol Obstet. 2013;287:919–936. doi: 10.1007/s00404-012-2626-6. [DOI] [PubMed] [Google Scholar]
- 10.Baden WF, Walker TA. Genesis of the vaginal profile: a correlated classification of vaginal relaxation. Clin Obstet Gynecol. 1972;15:1048–1054. doi: 10.1097/00003081-197212000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Petros PE. Dynamic Anatomy, Diagnosis of Connective Tissue damage, Surgery, in The Female Pelvic Floor, Function, Dysfunction and Management, according to the Integral Theory. 2nd edition. 2-4. Springer Heidelberg; 2006. pp. 48–138. [Google Scholar]
- 12.Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 13.Goeschen K. Role of Uterosacral Ligaments in the Causation and Cure of Chronic Pelvic Pain Syndrome. Pelviperineology. 2015;34:2–20. [Google Scholar]
- 14.Wren PA, Janz NK, Brubaker L, Fitzgerald MP, Weber AM, LaPorte FB, Wei JT. Reliability of health-related qualityof-life measures 1 year after surgical procedures for pelvic floor disorders. Am J Obstet Gynecol. 2005;192:780–788. doi: 10.1016/j.ajog.2004.10.603. [DOI] [PubMed] [Google Scholar]
- 15.Lawndy SSS, Withagen MI, Kluivers KB, Vierhout ME. Between hope and fear: patient's expectations prior to pelvic organ prolapse surgery. Int Urogynecol J. 2011;22:1159–1163. doi: 10.1007/s00192-011-1448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L. Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. Am J Obstet Gynecol. 2006;195:1837–1840. doi: 10.1016/j.ajog.2006.06.065. [DOI] [PubMed] [Google Scholar]
- 17.Swift SE. The distribution of pelvic organ support in a population of female subjects seen for routine gynecologic health care. Am J Obstet Gynecol. 2000;183:277–285. doi: 10.1067/mob.2000.107583. [DOI] [PubMed] [Google Scholar]
- 18.Barber MD, Maher C. Apical prolapse. Int Urogynecol J. 2013;24:1815–1833. doi: 10.1007/s00192-013-2172-1. [DOI] [PubMed] [Google Scholar]
- 19.Tseng LH, IIlene C, Chang SD, Lee CL. Modern role of sacrospinous ligament fixation for pelvic organ prolapse surgery: A systemic review. Taiwan J Obstet Gynecol. 2013;52:311–317. doi: 10.1016/j.tjog.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Denman MA, Gregory WT, Boyles SH, Smith V, Edwards SR, Clark AL. Reoperation 10 years after surgically managed pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2008;198:555.e1–5. doi: 10.1016/j.ajog.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Liang CC, Chang YL, Lin YH, Chang SD. Significance of bladder trabeculation in postmenopausal women with severe pelvic organ prolapse: clinical and urodynamic assessments. Menopause. 2013;20:813–817. doi: 10.1097/GME.0b013e31827f09a0. [DOI] [PubMed] [Google Scholar]
- 22.Miranne JM, Lopes V, Carberry CL, Sung VW. The effect of pelvic organ prolapse severity on improvement in overactive bladder symptoms after pelvic reconstructive surgery. Int Urogynecol J. 2013;24:1303–1308. doi: 10.1007/s00192-012-2000-z. [DOI] [PubMed] [Google Scholar]
- 23.Basu M, Wise B, Duckett J. Urgency resolution following prolapse surgery: is voiding important? Int Urogynecol J. 2013;24:1309–1313. doi: 10.1007/s00192-012-2010-x. [DOI] [PubMed] [Google Scholar]
- 24.Al-Zahrani AA, Gajewski J. Urodynamic findings in women with refractory overactive bladder symptoms. Int. J Urol. 2016;23:75–79. doi: 10.1111/iju.12954. [DOI] [PubMed] [Google Scholar]
- 25.De Boer TA, Kluivers KB, Withagen MIJ, Milani AL, Vierhout ME. Predictive factors for overactive bladder symptoms after pelvic organ prolapse surgery. Int Urogynecol J. 2010;21:1143–1149. doi: 10.1007/s00192-010-1152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]