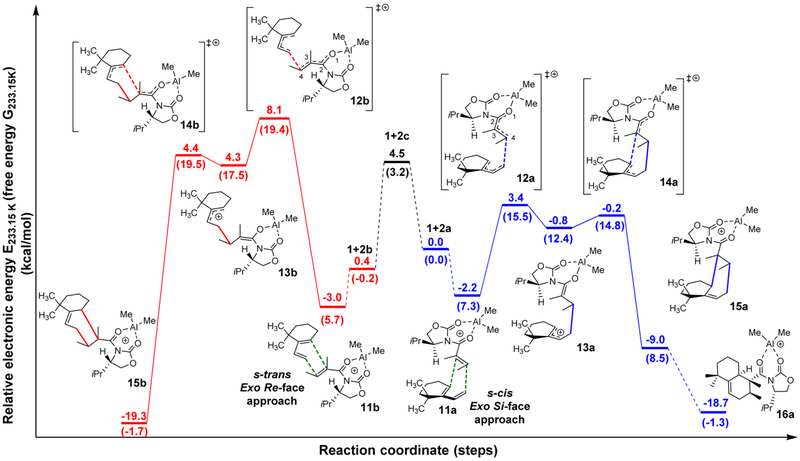
Figure 3.
DFT BP86/TZ2P calculated reaction coordinate for the Diels–Alder cycloaddition between diene 1 and dienophile 2. For these studies, the s-cis exo Si-face approach was chosen as the reference point. IRC analyses were performed on both transition states on the s-cis pathway (blue lines) and are connected with solid lines. The dashed lines connect the structures in which IRC was not performed, but was found to exist on the potential energy surface. (Relative electronic energies are given in kcal/mol, and relative Gibbs free energies are given in kcal/mol in parentheses.) The geometry of diene 1, aluminum-complexed s-cis conformer of the dienophile (2a), aluminum-complexed s-trans conformer of the dienophile (2b), and the transition state between 2a and 2b corresponding to the rotation along the C(2)–C(3) bond (2c) were optimized separately. The energies of the three states 1 + 2a, 1 + 2b and 1 + 2c are the sum of the corresponding diene and aluminum-complexed dienophile fragments (See Supporting Information).