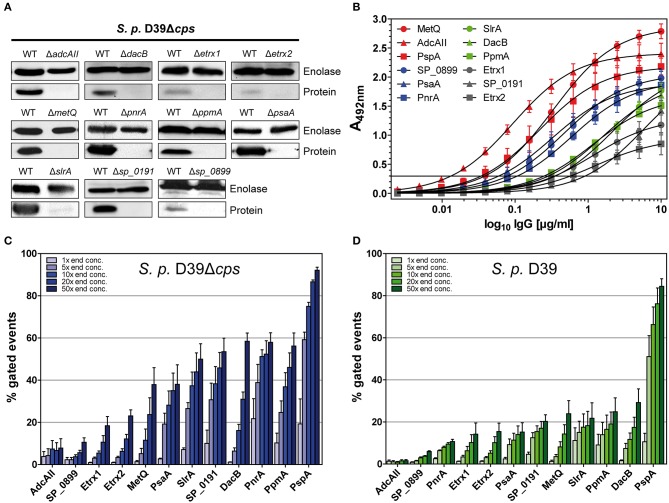
Figure 2.
Pneumococcal lipoproteins are highly abundant on the pneumococcal surface. (A) In immunoblots, the specificity of antisera derived from intraperitoneal immunisations of CD-1 mice (n = 6) with recombinant lipoproteins was assessed. Therefore, the wild-type strain S. pneumoniae D39Δcps and the corresponding isogenic lipoprotein deficient mutants (2 × 108 bacteria per lane) were used. Enolase was detected with a rabbit anti-enolase serum and served as a loading control. (B) IgG antibody titrations were performed by incubating equimolar amounts of recombinant proteins with serial dilutions of isolated polyclonal IgGs. Detection was carried out using a peroxidase-coupled goat anti-mouse IgG followed by incubation with OPD as a substrate and absorbance was measured at 492 nm. Titrations were performed at least three times and the error bars represent the SEM. (C,D) Using the equation for the hyperbolic regression curve (, Bmax, maximal binding; Kd, concentration for half maximal binding) an initial IgG concentration was calculated in the linear dynamic range. The polyclonal IgGs with equal contents of IgG specific for each lipoprotein were therefore applied to enable the comparison of their surface abundances. In a flow cytometric approach, D39Δcps (C) and D39 (D) were incubated with the appropriate calculated concentration of IgG and concentrations 5-, 10-, 20-, and 50-fold greater to analyse the surface abundance of the selected lipoproteins. Antibody binding was detected using a goat anti-mouse Alexa Fluor® 488-coupled secondary antibody. The percentage of positive gated events is depicted in the graphs, thereby indicating the proportion of wild-type bacteria positive for the binding of the respective anti-lipoprotein IgGs. The mean values of at least three independent experiments are shown, with error bars corresponding to SEM.