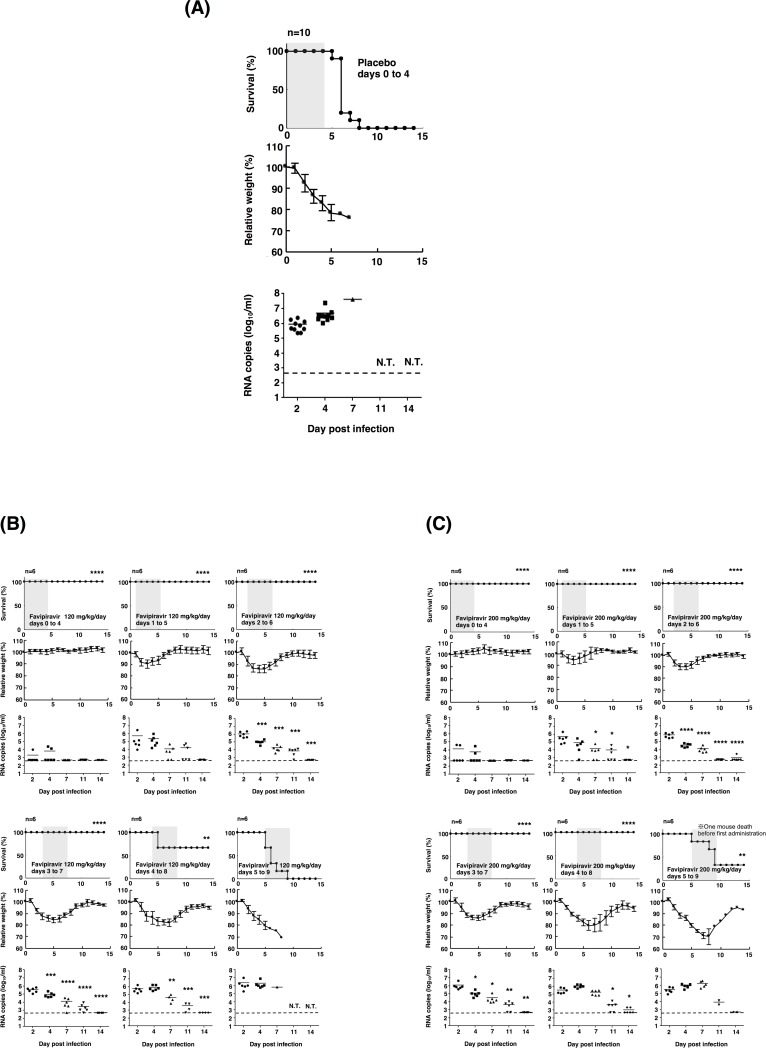
Fig 2. Effects of treatment with favipiravir against SFTSV infection in IFNAR−/− mice.
(A) Ten mice in the placebo control group were inoculated s.c. with 1.0 × 106 TCID50 of SFTSV strain SPL010. Control mice received 0.5% (w/v) methylcellulose solution via the p.o. route. (B, C) Six mice in each group were inoculated s.c. with 1.0 × 106 TCID50 of SFTSV strain SPL010. Mice were treated with favipiravir at a dose of 120 mg/kg/day (B, 60 mg/kg/BID, p.o.) or 200 mg/kg/day (C, 100 mg/kg/BID, p.o.). Treatment was commenced at 1 h or 1, 2, 3, 4, or 5 days post infection. Favipiravir was administered twice daily p.o. using a stomach probe until death or for 5 days as indicated in the upper columns (shaded in gray with survival curves). Survival was determined using Kaplan–Meier analysis and GraphPad Prism6 (GraphPad Software) and shown in the upper columns. Relative weights are shown as means with standard deviations (middle columns). SFTSV RNA levels in blood samples collected at 2, 4, 7, 11, or 14 days post infection were determined by quantitative RT-PCR assays (lower columns). One way ANOVA with Bonferroni’s multiple comparison test was used to determine statistical significance. Dashed lines indicate the detection limits of the assay in blood samples. Significance was determined in comparison to the results of the placebo group (for survivals) or Day 2 blood samples (for RNA copies): ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; * P < 0.05; N.T., not tested.