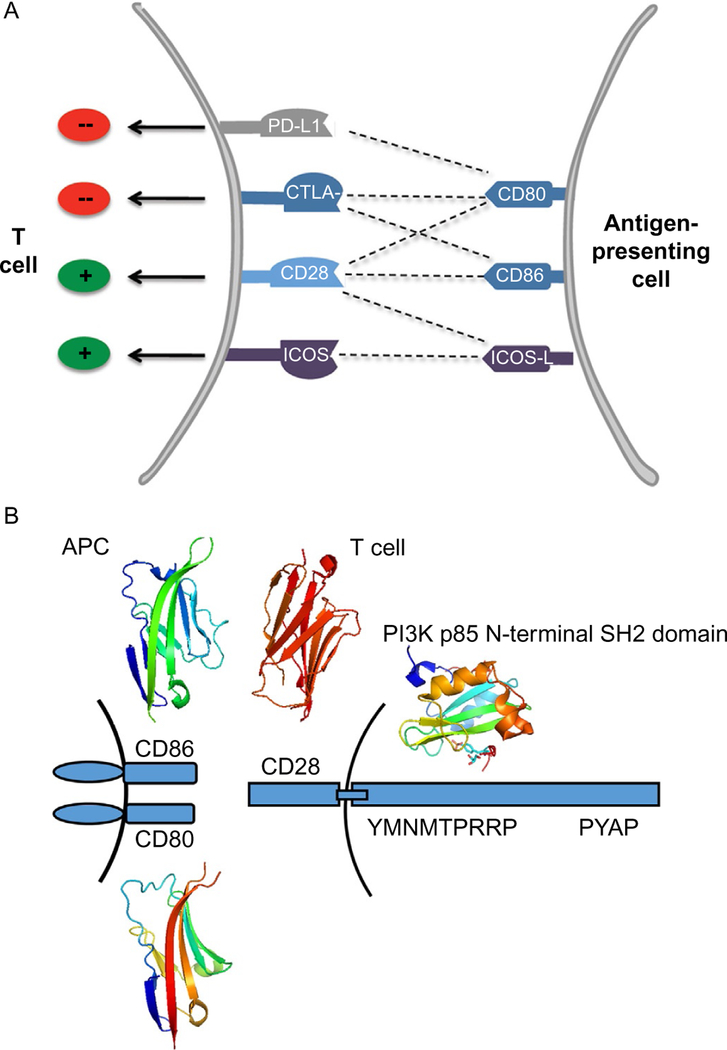
Fig. 17.
(A) Complexities of CD28 costimulatory pathways. The CD28 costimulatory receptor can be ligated by CD80, CD86, and ICOS-L (B7–H2). The CTLA-4 coinhibitor competes with CD28 for binding to CD80 and CD86. However, CD80 can also bind to PD-L1 (B7–H1) and deliver a coinhibitory signal. ICOS competes with CD28 for binding to B7–H2. (B) Schematic representation of PPI interactions of CD28 with CD80/CD86. CD28 cytoplasmic domain motifs YMNM and PYAP that are important in signaling are indicated. Crystal structures of domains of CD28 (1YJD), CD80 (4RWH), CD86 (1NCN), and p53 (5GJI) domain important in binding to CD28 signaling are shown. Panel (A): Reproduced with permission from Ford, M. L., Adams, A. B., & Pearson, T. C. (2014). Targeting co-stimulatory pathways: Transplantation and autoimmunity. Nature Reviews Nephrology, 10(1), 14–24. https://doi.org/10.1038/nrneph.2013.183. Copyright (2013) Nature Publishing Group.