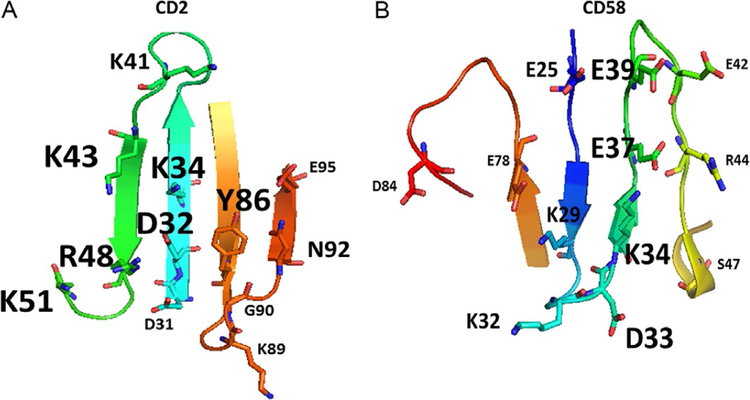
Fig. 5.
The structure of PPI domains of (A) CD2 and (B) CD58 with amino acids that form hydrogen bonding, salt bridges, and hydrophobic interaction (PDB ID 1QA9). Mutation studies suggested that the affinity of interaction between the two proteins is reduced when alanine is replaced by some of the residues in the proteins (Kim et al., 2001; Wang et al., 1999). Residues that have the most effect on affinity of binding are represented by large-sized letters for amino acid labels. Residues that partially affect the affinity are represented with medium-sized letters. Residues that do not affect the affinity by replacement with alanine are represented with small-sized letters.