Abstract
A simple, short, and rugged LC-MS/MS method for the simultaneous determination of tenofovir, emtricitabine, elvitegravir and rilpivirine was developed and validated. Dried blood spots were prepared with 25 μL of spiked whole blood. A 3 mm punch was extracted with methanol containing labeled internal standards. Ten microliter was injected into LC-MS/MS using isocratic mobile phase composed of 0.1% formic acid in water and 0.1% formic acid in acetonitrile (45: 55 v/v) at a flow rate of 0.25 mL/min. The method was validated in the range of 10 to 2000 ng/mL for all four analytes. The intra-assay accuracy (%RE) of the method was −4.73 to 4.78, 1.35 to 2.89, −8.89 to −0.49 and −1.40 to 1.81 for tenofovir, emtricitabine, elvitegravir and rilpivirine respectively. The inter-assay accuracy was within ± 15% of nominal and precision (%CV) was < 15%. The hematocrit effect on quantification was non-significant at the tested hematocrit levels (35 to 70%). The DBS method had shown good agreement with plasma method, hence can be used as an alternative to plasma method.
Keywords: Antiretrovirals, mass spectrometry, dried blood spot, plasma
1. Introduction
Combination antiretroviral therapy (cART) has proven to be effective in prevention and treatment of HIV (Cihlar & Fordyce, 2016). USFDA approved single-tablet combination antiretroviral drugs for the treatment of HIV comprised mainly of tenofovir (TFV) prodrug, and emtricitabine (FTC) (NIH, Drugs that fight HIV-1, 2016). Truvada™, which contains tenofovir disoproxil fumarate (TDF) and FTC, is the only FDA-approved medicine for pre-exposure prophylaxis (PrEP) for HIV. Combinations of TFV prodrug, FTC and elvitegravir (EVG) or rilpivirine (RPV) have proven to be efficacious and are approved for HIV treatment (NIH, Drugs that fight HIV-1, 2016).
Adherence to treatment is one of the major issues with antiretroviral therapy (ART). To evaluate patient adherence, therapeutic drug monitoring from patients has been advocated to determine the concentration of drug in systemic circulation (Landovitz et al., 2017). Human trials of antiretroviral drugs for HIV treatment in low-income zones like West Africa has demonstrated good efficacy and concluded that a dried blood spot (DBS) method might improve the assessment of ART failure (de Truchis et al., 2016). A simple analytical method to quantitate drugs with small sample volume is highly desirable, that make it cost effective and socio-economically acceptable method. Several assays have been reported for the quantification of TFV, FTC, EVG, and RPV as an individual assay/combinations of these drugs in plasma (Aouri et al., 2013, Delahunty et al., 2009, Gomes et al., 2008, Illamola et al., 2016, Parsons & Marzinke, 2016, Penchala et al., 2016, & Prathipati et al., 2016). Since plasma processing is tedious, time-consuming and requires high sample volume, dried blood spot methodology is advantageous (Li & Tse, 2010). DBS methods for simultaneous quantification of TFV and FTC were previously reported (Waitt et al., 2017, & Zheng et al., 2014) but a simultaneous quantification of TFV, FTC, EVG, and RPV has not been reported yet.
Our group has been working on different combinations of TFV prodrug, FTC, and EVG or RPV nanoformulations for sustained release to determine the pharmacokinetics (PK) in humanized mice model. We have previously reported a high throughput method for simultaneous quantification of these analytes along with dolutegravir in plasma and mouse tissues (Prathipati et al., 2016). Since we are working on the small animal model, the sample volume is a challenge for bioanalysis, especially for PK and efficacy studies. It would be ideal to use the same sample to assay drug concentrations and viral load, we have developed and validated a simple DBS method to accommodate both the assays using the same sample. The validated method was applied to PK study.
2. Materials and methods
2.1. Chemicals and reagents
TFV reference standard was purchased from United States Pharmacopeia, (Rockville, MD). FTC, EVG and RPV reference standards were purchased from Sequoia, Pangbourne, United Kingdom. Labelled internal standards (TFV-d6, FTC-13C,15N2, EVG-d6 and RPV-d6) were purchased from Toronto Research Chemicals Inc., Canada. Milli-Q water was obtained from in-house Milli-Q water purification system, Millipore, USA. LC-MS grade methanol, acetonitrile, and formic acid were purchased from Fisher Scientific, USA. Whatman 903 protein saver DBS cards were purchased from GE Healthcare, Cardiff, UK.
2.2. Instrumentation
An Exion HPLC system (Applied Biosystems, Foster City, CA, USA) coupled with AB Sciex API 5500 Q Trap with an electrospray ionization (ESI) source (Applied Biosystems, Foster City, CA, USA) was used. The LC-MS/MS system was controlled by Analyst 1.6.3 software.
2.3. Preparation of calibration curve and quality control standards
FTC, EVG, RPV and internal standards (TNF-d6, FTC-13C,15N2, EVG-d6 and RPV-d6) stock solutions were prepared in methanol. TFV stock solution was prepared in water:methanol (80:20, v/v). All stock solutions were prepared at 1 mg/mL concentration. The working standard solutions were prepared in water:methanol (50:50, v/v) from stock solution and used to prepare the calibration and quality control samples. Internal standard (IS) spiking solution was prepared in methanol.
An eight-point calibration curve was prepared by spiking the previously screened blank human blood with a corresponding working standard solution not exceeding 5% v/v. The calibration curve ranged from 10 to 2000 ng/mL for all the analytes. Similarly, quality control samples were prepared at four concentrations 10 (LLQC) 30 (LQC), 900 (MQC) and 1800 ng/mL (HQC). DBS samples were prepared by spotting 25 μL of whole blood contained either standard or QC onto Whatman 903 protein saver cards. After spotting, the cards were allowed to dry overnight. Once dried, the cards were placed in plastic bags with desiccant sachets and stored at room temperature, −20°C and −80°C.
2.4. Sample processing
A 3 mm diameter disk was punched out from the blood spots for extraction and placed into a microcentrifuge tube. An aliquot of 500 μL of methanol containing internal standards was added to each tube. Samples were sonicated for 15 min and centrifuged for 5 min at 14000 RPM. An aliquot of 450 μL supernatant was transferred into a tube and evaporated using nitrogen to dryness at 40°C. The dried residue was reconstituted with 100 μL 50% acetonitrile, centrifuged, transferred into autosampler vials and 10 μL was injected into LC-MS/MS for analysis.
2.5. Liquid chromatography and mass spectrometry conditions
We used the similar chromatographic conditions that we previously reported (Prathipati et al., 2016). A Restek Pinnacle DB Biph (2.1mm × 50mm, 5μm) column with isocratic mobile phase consisting of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B) (45:55 v/v) at a flow rate of 0.250 mL/min was used. The API 5500 Q Trap mass spectrometer was operated in positive multiple reaction monitoring (MRM) mode. The mass spectrometer transitions (precursor/product) were: TFV (288.0/176.2), FTC (248.1/130.1), EVG (448.2/344.1), RPV (367.2/195.1), TFV-IS (293.9/182.3), FTC-IS (251.1/133.1), EVG-IS (454.2/350.1) and RPV-IS (373.2/195.1). The source temperature, ion spray voltage, and gas pressures were optimized through flow injection analysis (FIA) by infusing mobile phase using LC. The source temperature, ion spray voltage and gas pressures (GS1 and GS2) were set at 550°C, 5500V, 55 and 60 psi respectively. The retention times were 0.6, 0.6, 2.0 and 1.98 for TFV, FTC, EVG and RPV respectively. The total run time for each sample was 4 min.
2.6. Method Validation
The fundamental parameters for bioanalytical method validation in plasma/tissue are selectivity, matrix effect, accuracy, precision, recovery, dilution integrity, and stability. As such there are no guidelines for the validation of DBS method, however, the most important factors that have potential to affect DBS outcome are hematocrit (HCT), spot volume and punch location which should be evaluated for uncompromised performance of the DBS method. A full validation was performed in accordance with FDA guidelines (US Department of Health and Human Services, 2001) and widely accepted reported methodologies (Li & Tse, 2010, & Spooner, 2009).
2.6.1. Selectivity
Selectivity was evaluated to determine the interference arising from matrix and DBS card by analyzing six different sources of human blood samples spotted on DBS cards. The method was considered selective when the peak area at the retention time of analyte(s) was less than 20% of the LLOQ of respective analyte(s).
2.6.2. Matrix effect and extraction recovery
Three sets of samples were prepared. Set-A: neat standards of LQC, MQC and HQC were prepared in 50% acetonitrile that has the same concentration as that of respective post extracted QC sample. Set-B: six replicates each of blank plasma spiked at LQC, MQC and HQC level were extracted. Set-C: eighteen blank samples were extracted and reconstituted with neat standards (Set-A) at LQC and MQC and HQC level six each. For matrix effect peak area from Set-C were compared with Set-A. For relative extraction recovery peak areas of Set-C were compared with Set-B. For absolute extraction recovery (process efficiency) peak areas of Set-B were compared with Set-A. For the preparation of neat standards, the blood volume of 3 mm disk was determined by surface area approach. The surface area of 25 μL dried blood spot (average diameter was 9 mm) and 3 mm spot were calculated and blood volume (2.78 μL for 3 mm spot) was estimated. An estimated blood volume was used to prepare neat standard to match post extracted sample concentration.
2.6.3. Calibration curves
Linearity was evaluated by analyzing three calibration curves having eight non-zero standards, zero sample (with IS) and blank (No IS) on different days in the range 10–2000 ng/mL for all the analytes. The linear regression with 1/x2 weighting factor was used for back-calculation of analyte concentrations. The acceptance criteria for calculated concentration was ±15% of the theoretical value, except for LLOQ where ±20% was considered acceptable. The acceptance criteria for correlation coefficient was ≥ 0.9900.
2.6.4. Precision and accuracy
Intra-assay and inter-assay precision and accuracy were determined using six replicates each at four concentration levels. Three different batches were run along with calibration curves. The inter-assay and intra-assay precision were expressed as coefficient of variation (%CV) and accuracy was expressed as a relative error (i.e. % deviation between theoretical and measured concentrations). The acceptance criteria for precision and accuracy were ≤15% and ±15% respectively.
2.6.5. Dilution integrity
Dilution integrity was evaluated by extracting six replicates of DBS spot spiked with 4000 ng/mL and blank samples with IS. Post extraction, spiked sample was diluted with blank (with IS) at 1:3 and 1:9 ratios and dilution factors were applied to back-calculated concentrations.
2.6.6. Influence of hematocrit
Three different hematocrit (35, 50 and 70%) blood samples were prepared in-house by adding or removing plasma to the whole blood. Hematocrits were measured using a microhematocrit centrifuge and capillary reader (Damon, MS). These blood samples were spiked with LQC and HQC, spotted, dried and extracted in triplicate. The back-calculated concentrations of these QC’s were measured from calibration curve made of HCT 50%. The relative error of ±15% and precision of ≤15% was considered acceptable.
2.6.7. Influence of spot volume
Fifty microliter spots at LQC and HQC were made and extracted in triplicate. The concentrations of these QC’s were measured from calibration curve made of the 25μL spot. The relative error of ±15% and precision of ≤15% was considered acceptable.
2.6.8. Influence of punch location
Punches from edges of LQC and HQC were extracted in triplicate and compared the peak area ratios with punches from the center of the spot. Also, concentrations were back-calculated from calibration curve made of center punched. The % difference/relative error within ±15% and precision of ≤15% were considered acceptable.
2.6.9. Stability experiments
Stability of analytes was tested to determine the effect of conditions that were expected to be encountered during sample handling and analysis. For benchtop stability, spiked dried blood spots were kept on benchtop at room temperature for 20 h before processing. For freeze-thaw stability, spiked quality control samples were exposed to 3 freeze thaw cycles before processing. For processed sample stability, extracted quality control samples were transferred to autosampler vials and kept at room temperature for 26 h before analysis. For long-term stability evaluation, quality control samples stored at −80°C for 4 months were used. Reinjection reproducibility of the assay was determined by reinjecting the accepted precision and accuracy run. Six replicates of LQC and HQC at each level were tested for all stability experiments. The stability sample concentrations were back-calculated from freshly spiked and processed calibration curve. The relative error of ±15% and precision of ≤15% was considered acceptable for stability QC’s.
2.7. Application
A pharmacokinetic study in female hu-NSG mice (Jackson Laboratory, Bar Harbor, MA) administered SubQ with FTC+EVG NPs (200 mg/kg) in 1 mL D5W. At specific times (1, 4 and 12 hours post-administration), mice (n=3/time point) were euthanized. Blood was collected in sodium heparin Vacutainers and 25 μL was spotted on to Whatman 903 protein saver card. DBS cards were allowed to dry overnight. Once dried, the cards were placed in plastic bags with desiccant sachets and stored at −80°C until analysis. Plasma was separated and tissues were collected and stored at −80°C for FTC and EVG concentration measurement by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The non-compartmental analysis was performed using WinNonlin (Certara Inc., Princeton, NJ).
3. Results and Discussion
3.1. Method Development
The primary objective of this method is to develop a sensitive and rugged method for the simultaneous quantification of selected antiretroviral drugs to support pharmacokinetic and in-vivo efficacy study of nanoformulated combination antietroviral drugs in mice. It is ideal to measure the drug concentration and viral load from the same sample. Due to the limited volume of the blood sample from mice, it is challenging to use the same sample for measurement of drug concentration and viral load. Hence we have developed, validated a DBS method and successfully applied it to a pharmacokinetic study in mice. We have previously reported a simultaneous method for quantification these analytes along with dolutegravir in plasma and tissues (Prathipati et al., 2016). The similar chromatographic separation was utilized for this method. Since the sample volumes used for DBS are very low compared to plasma method, a higher sensitive mass spectrometer was used.
Since the chromatographic and mass spectrometric conditions were optimized to yield better sensitivity, extraction optimization was carried out with different extraction solvents like acetonitrile, methanol, acidified acetonitrile/methanol and a mixture of water-acetonitrile/methanol were tested. Among all the tested extraction solvents, extraction efficiency was good with methanol. The volume of extraction solvent and duration of sonication were also optimized to obtain better recovery. For all the analytes desired sensitivity was achieved with 10μL injection volume and less runtime of 4 minutes.
3.2. Method validation
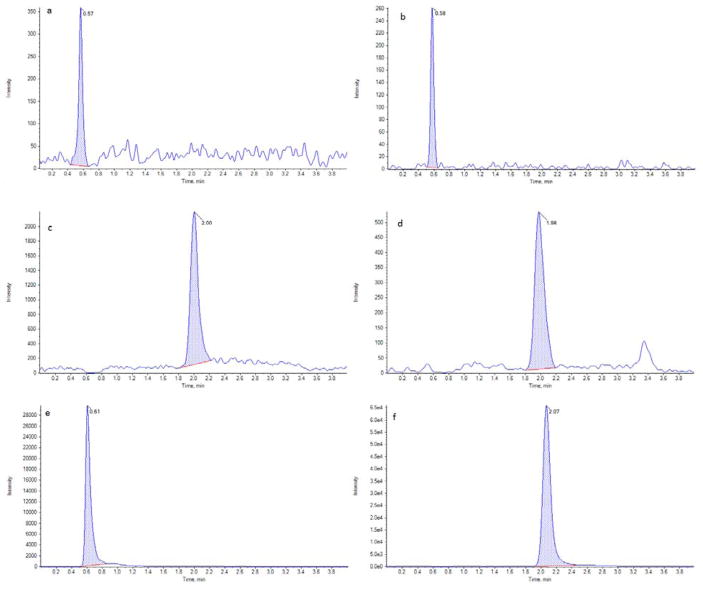
The method is selective for analytes tested as the interference observed at the retention times of analytes in blank samples was less than 20% of lower limit of quantification. The representative chromatograms of LLOQ and unknown sample are presented in Fig. 1. The mean % matrix effects were 103.21%, 97.44%, 97.11% and 99.62% with %CV values between 2.1 to 6.9% and mean relative recoveries were 50.1%, 56.0%, 76.1% and 74.8% with %CV values between 3.4 to 8.3% for TFV, FTC, EVG and RPV respectively. The mean absolute recoveries (process efficiencies) were for TFV, FTC, EVG and RPV were 52.4%, 52.8%, 73.4% and 74.1% with %CV values between 2.2 to 7.6%. The low absolute and relative recoveries even without significant matrix effect could be attributed to drug binding to cellulose and/or over estimation of blood volume in 3 mm punch for neat solution preparation.
Fig. 1.
Representative chromatograms of LLOQ and unknown sample. Extracted LLOQ of TFV (a), FTC (b), EVG (c) and RPV (d). Extracted unknown of FTC (e) and EVG (f).
The three calibration curves had a correlation coefficient greater than 0.9961 for all the four analytes. The mean relative error for all the calibration standards was in the range of −8.9 to 9.7, −8.1 to 7.6, −6.1 to 7.7, and −3.3 to 5.6 for TFV, FTC, EVG and RPV respectively. The results of calibration curve are summarized in Table 1. The results of inter and intra-assay precision and accuracy for all the four analytes were within the acceptable limits, %CV≤15% and %RE, 100±15% at each concentration level. The results of precision and accuracy are presented in Table 2. The ability to dilute samples above the upper limit of quantification was verified by diluting samples four-fold and ten-fold, the bias and precision values were less than the pre-defined acceptance limits of 15%.
Table 1.
Summary of calibration curves
| TFV | FTC | EVG | RPV | |
|---|---|---|---|---|
| Calibration curve range | 10 to 2000 ng/mL | |||
| Inter-assay Accuracy (% RE) (n=3) | −8.9 to 9.7 | −8.1 to 7.6 | −6.1 to 7.7 | −3.3 to 5.6 |
| Inter-assay Precision (% CV) (n=3) | 0.5 to 8.1 | 0.07 to 8.2 | 0.19 to 4.2 | 1.11 to 4.8 |
| Correlation coefficient (r) | > 0.9961 | > 0.9978 | > 0.9988 | > 0.9985 |
Table 2.
Summary of precision and accuracy of DBS method.
| TFV | FTC | EVG | RPV | |
|---|---|---|---|---|
| LLQC | 10 ng/mL | |||
| Intra-assay Accuracy (% RE) (n=6) | 4.19 | 1.92 | −0.49 | 1.81 |
| Intra-assay Precision (% CV) (n=6) | 4.43 | 7.84 | 8.47 | 6.54 |
| Inter-assay Accuracy (% RE) (n=18) | −0.33 | 2.88 | 4.09 | 4.35 |
| Inter-assay Precision (% CV) (n=18) | 7.68 | 8.11 | 10.6 | 4.22 |
| LQC | 30 ng/mL | |||
| Intra-assay Accuracy (% RE) (n=6) | 4.78 | 2.89 | −3.46 | 1.68 |
| Intra-assay Precision (% CV) (n=6) | 3.52 | 6.55 | 7.52 | 5.45 |
| Inter-assay Accuracy (% RE) (n=18) | 1.25 | 5.76 | −0.12 | 2.02 |
| Inter-assay Precision (% CV) (n=18) | 7.24 | 8.03 | 6.65 | 4.19 |
| MQC | 900 ng/mL | |||
| Intra-assay Accuracy (% RE) (n=6) | −4.73 | 1.35 | −8.89 | −1.40 |
| Intra-assay Precision (% CV) (n=6) | 6.41 | 5.02 | 5.01 | 4.03 |
| Inter-assay Accuracy (% RE) (n=18) | −6.46 | −4.22 | −9.04 | −4.01 |
| Inter-assay Precision (% CV) (n=18) | 6.63 | 7.62 | 6.75 | 6.67 |
| HQC | 1800 ng/mL | |||
| Intra-assay Accuracy (% RE) (n=6) | −2.57 | 2.23 | −3.58 | 0.12 |
| Intra-assay Precision (% CV) (n=6) | 7.14 | 7.41 | 1.19 | 2.40 |
| Inter-assay Accuracy (% RE) (n=18) | −5.12 | 0.56 | −3.21 | 0.63 |
| Inter-assay Precision (% CV) (n=18) | 7.64 | 4.82 | 2.02 | 3.15 |
DBS samples stored on bench top for 20 h, freeze-thaw (3 cycles) and processed samples stored at ambient temperature were stable for 26 h. No significant difference in %RE was observed on reinjection of accepted analytical precision and accuracy run. Long-term stability samples stored at −20°C and −80°C for 4 months had % difference within ± 7.2% of control (fresh) samples. All the stability sample results, % RE were within 85 to 115% and %CV≤15%.
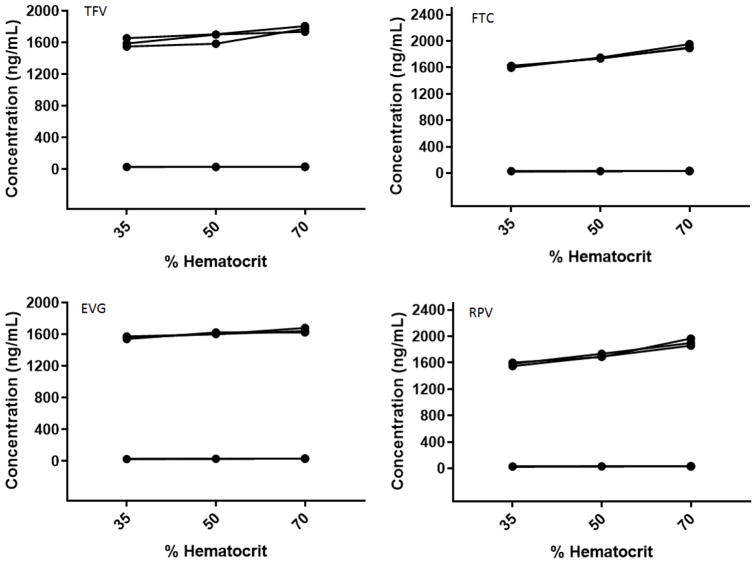
The DBS specific validation experiments, spot volume, punch location and influence of hematocrit yielded valuable information that could translate the applicability of this method for in-vivo studies. The mean calculated concentrations (n=6) of tested quality control standards for all the analytes at both the concentrations (LQC and HQC) were within the predefined acceptance limits for spot volume and punch location. The concentrations of quality controls were increasing with increase in hematocrit for all the analytes (Fig. 2). The blood-to-plasma ratios of TFV, FTC, EVG and RPV are 0.58, 1, 0.73 and 0.65 (De Sousa Mendes, 2016, Center for Drug Evaluation and Research, Application Number: 203100Orig1s000, & USFDA Label, Reference ID: 4184673). Theoretically, drugs with blood-to-plasma ratio in the range 0.55–0.60 with high protein binding do not partition into erythrocytes (Emmons, 2010). The less protein binding of tenofovir and emtricitabine and partitioning to blood cells could be the underlying cause for the increase in concentration with increase in hematocrit. Whereas elvitegravir and rilpivirine have high protein binding, blood plasma ratios less than 1 and still has a non-significant effect on quantification at the hematocrit levels tested. It is worth noting to take the difference in spot size with HCT, blood-to-plasma ratio and RBC-to-plasma ratios into consideration to understand the increase of drug concentrations with increase in HCT for these drugs and also in general for DBS method development. The results of hematocrit are presented in Table 3.
Fig. 2.
Hematocrit effect on TFV, FTC, EVG and RPV. (Black dots represent the measured concentration of LQC and HQC at 35, 50 and 70% hematocrit values).
Table 3.
Hematocrit effect analysis
| Analyte | Parameter | % Hematocrit | % Hematocrit | ||||
|---|---|---|---|---|---|---|---|
| 35 | 50 | 70 | 35 | 50 | 70 | ||
| LQC (10 ng/mL) | HQC (1800 ng/mL) | ||||||
| TFV | Mean Concentration (ng/mL) (n=3) | 28.94 | 30.96 | 31.92 | 1596.60 | 1663.20 | 1772.40 |
| Accuracy (%RE) | −3.53 | 3.20 | 6.40 | −11.30 | −7.60 | −1.53 | |
| Precision (% CV) | 3.65 | 2.14 | 0.98 | 2.80 | 3.37 | 1.62 | |
| FTC | Mean Concentration (ng/mL) (n=3) | 28.67 | 31.85 | 33.11 | 1613.40 | 1743.60 | 1917.60 |
| Accuracy (%RE) | −4.43 | 6.17 | 10.37 | −10.37 | −3.13 | 6.53 | |
| Precision (% CV) | 5.65 | 0.65 | 1.10 | 0.79 | 0.34 | 1.39 | |
| EVG | Mean Concentration (ng/mL) (n=3) | 27.48 | 28.87 | 32.21 | 1557.00 | 1609.80 | 1648.20 |
| Accuracy (%RE) | −8.40 | −3.77 | 7.37 | −13.50 | −10.57 | −8.43 | |
| Precision (% CV) | 1.29 | 3.43 | 1.94 | 0.85 | 0.61 | 1.41 | |
| RPV | Mean Concentration (ng/mL) (n=3) | 28.89 | 31.90 | 33.43 | 1578.00 | 1705.68 | 1908.00 |
| Accuracy (%RE) | −3.71 | 6.32 | 11.42 | −12.33 | −5.24 | 6.00 | |
| Precision (% CV) | 1.91 | 1.20 | 3.40 | 1.42 | 1.23 | 2.30 | |
3.3. Application and correlation of DBS vs Plasma methods
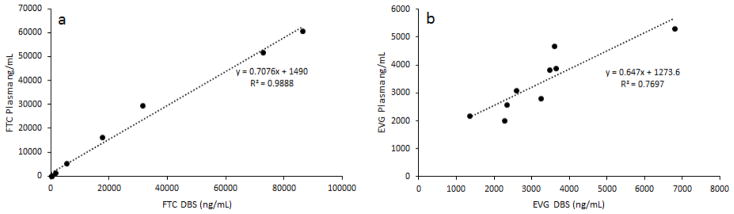
This method was successfully applied to study the pharmacokinetic profile of antiretroviral drugs formulated into nanoparticles. The correlation between DBS and plasma methods was studied using the same blood sample for DBS and plasma. Plasma samples were analyzed using the previously published method (Prathipati et al., 2016) with the exception of instrument used, API 5500 Q Trap was employed. Linear regression analysis (Fig. 3) has demonstrated good agreement between DBS and plasma for FTC and EVG. The coefficients of determinations (r2) were 0.9888 and 0.7697 for FTC and EVG respectively.
Fig. 3.
Correlation of DBS vs plasma concentration of FTC (a) and EVG (b).
3.4. Significance over other reported methods
There are two reported methods for quantification of tenofovir and emtricitabine in dried blood spots (Waitt et al., 2017, & Zheng et al., 2014). Both the methods have longer time run time (≥6 mins) than our method (4 mins). Zheng et al. has used isocratic mobile phase with high aqueous content (99.5% v/v) and Waitt et al. has used gradient program starting at 95% v/v aqueous phase, whereas we have used isocratic mobile phase with 45 % aqueous which is highly compatible for mass spectrometer. We have quantified elvitegravir and rilpivirine, which is unique compared to reported methods.
4. Conclusion
The developed method for simultaneous quantification of TFV, FTC, EVG, and RPV in DBS was demonstrated to be accurate, precise and rugged. The DBS specific validation parameters like hematocrit, spot volume, and punch location have demonstrated that this method can be used as an alternative to plasma. The correlation between DBS and plasma was demonstrated for emtricitabine and elvitegravir in unknown samples. This method can be conveniently applied to current human trials on a combination of these drugs, especially in low-income countries.
Acknowledgments
This work was supported by NIH grant RO1 AI117740-01 to C.J.D. The Animal Research Facility is supported by Grant Number G20RR024001 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Abbreviations
- ART
Antiretroviral therapy
- D5W
5% dextrose in water
- DBS
Dried blood spot
- EVG
Elvitegravir
- FTC
Emtricitabine
- HCT
Hematocrit
- HIV
Human immunodeficiency virus
- HPLC
High performance liquid chromatography
- HQC
High quality control
- IS
Internal standard
- LC-MS/MS
Liquid chromatography-tandem mass spectrometry
- LLOQ
Lower limit of quantification
- LLQC
Lower limit quality control
- LQC
Low quality control
- MQC
Mid quality control
- NP
Nanoparticle
- PK
Pharmacokinetic
- PrEP
Pre-exposure prophylaxis
- QC
Quality control
- RPV
Rilpivirine
- TDF
Tenofovir disoproxil
- TFV
Tenofovir
References
- Aouri M, Calmy A, Hirschel B, Telenti A, Buclin T, Cavassini M, Rauch A, Decosterd LA. A validated assay by liquid chromatography-tandem mass spectrometry for the simultaneous quantification of elvitegravir and rilpivirine in HIV positive patients. Journal of Mass Spectrometry. 2013;48:616–625. doi: 10.1002/jms.3200. [DOI] [PubMed] [Google Scholar]
- Center for Drug Evaluation and Research. Clinical Pharmacology and Biopharmaceutics Review(s) Application Number: 203100Orig1s000. [Google Scholar]
- Cihlar Tomas, Fordyce Marshall. Current status and prospects of HIV treatment. Current Opinion in Virology. 2016;18:50–56. doi: 10.1016/j.coviro.2016.03.004. [DOI] [PubMed] [Google Scholar]
- De Sousa Mendes M, Hirt D, Vinot C, Valade E, Lui G, Pressiat C, Bouazza N, Foissac F, Blanche S, Le MP, Peytavin G, Treluyer JM, Urien S, Benaboud S. Prediction of human fetal pharmacokinetics using ex vivo human placenta perfusion studies and physiologically based models. British Journal of Clinical Pharmacology. 2016;81:646–657. doi: 10.1111/bcp.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Truchis P, Le MP, Daou M, Madougou B, Nouhou Y, Moussa Saley S, Sani A, Adehossi E, Rouveix E, Saidou M, Peytavin G, Delaugerre C. High efficacy of first-line ART in a West African cohort, assessed by dried blood spot virological and pharmacological measurements. Journal of Antimicrobial Chemotherapy. 2016;71:3222–3227. doi: 10.1093/jac/dkw286. [DOI] [PubMed] [Google Scholar]
- Delahunty T, Bushman L, Robbins B, Fletcher CV. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. Journal of Chromatography B. 2009;877:1907–1914. doi: 10.1016/j.jchromb.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons G, Rowland M. Pharmacokinetic considerations as to when to use dried blood spot sampling. Bioanalysis. 2010;2:1791–1796. doi: 10.4155/bio.10.159. [DOI] [PubMed] [Google Scholar]
- Gomes NA, Vaidya VV, Pudage A, Joshi SS, Parekh SA. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) method for simultaneous determination of tenofovir and emtricitabine in human plasma and its application to a bioequivalence study. Journal of Pharmaceutical and Biomedical Analysis. 2008;48:918–926. doi: 10.1016/j.jpba.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Illamola SM, Valade E, Hirt D, Dulioust E, Zheng Y, Wolf JP, Treluyer JM. Development and validation of a LC-MS/MS method for the quantification of tenofovir and emtricitabine in seminal plasma. Journal of Chromatography B. 2016;1033–1034:234–241. doi: 10.1016/j.jchromb.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Landovitz RJ, Beymer M, Kofron R, Amico KR, Psaros C, Bushman L, Anderson PL, Flynn R, Lee DP, Bolan RK, Jordan WC, Tseng CH, Dierst-Davies R, Rooney J, Wohl AR. Plasma Tenofovir Levels to Support Adherence to TDF/FTC Preexposure Prophylaxis for HIV Prevention in MSM in Los Angeles, California. Journal of Acquired Immune Deficiency Syndrome. 2017;76:501–511. doi: 10.1097/QAI.0000000000001538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Tse FL. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomedical Chromatography. 2010;24:49–65. doi: 10.1002/bmc.1367. [DOI] [PubMed] [Google Scholar]
- National Institute of Allergy and Infectious Diseases. A reference guide for prescription HIV-1 medications. 2016. Drugs that fight HIV-1. (NIH Publication No. 16-7628) [Google Scholar]
- Parsons TL, Marzinke MA. Development and validation of a liquid chromatographic-tandem mass spectrometric method for the multiplexed quantification of etravirine, maraviroc, raltegravir, and rilpivirine in human plasma and tissue. Journal of Pharmaceutical and Biomedical Analysis. 2016;131:333–344. doi: 10.1016/j.jpba.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Penchala SD, Fawcett S, Else L, Egan D, Amara A, Elliot E, Challenger E, Back D, Boffito M, Khoo S. The development and application of a novel LC-MS/MS method for the measurement of Dolutegravir, Elvitegravir and Cobicistat in human plasma. Journal of Chromatography B. 2016;1027:174–180. doi: 10.1016/j.jchromb.2016.05.040. [DOI] [PubMed] [Google Scholar]
- Prathipati PK, Mandal S, Destache CJ. Simultaneous quantification of tenofovir, emtricitabine, rilpivirine, elvitegravir and dolutegravir in mouse biological matrices by LC-MS/MS and its application to a pharmacokinetic study. Journal of Pharmaceutical and Biomedical Analysis. 2016;129:473–481. doi: 10.1016/j.jpba.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner N, Lad R, Barfield M. Dried blood spots as a sample collection technique for the determination of pharmacokinetics in clinical studies: considerations for the validation of a quantitative bioanalytical method. Analytical Chemistry. 2009;81:1557–1563. doi: 10.1021/ac8022839. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine. Guidance for Industry: Bioanalytical Method Validation. 2001 May; Available at: https://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf.
- USFDA Label. Highlights of Prescribing Information. JULUCA; Reference ID: 4184673. [Google Scholar]
- Waitt C, Diliiy Penchala S, Olagunju A, Amara A, Else L, Lamorde M, Khoo S. Development, validation and clinical application of a method for the simultaneous quantification of lamivudine, emtricitabine and tenofovir in dried blood and dried breast milk spots using LC-MS/MS. Journal of Chromatography B. 2017;1060:300–307. doi: 10.1016/j.jchromb.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JH, Guida LA, Rower C, Castillo-Mancilla J, Meditz A, Klein B, Klein B, Kerr BJ, Langness J, Bushman L, Kiser J, Anderson PL. Quantitation of tenofovir and emtricitabine in dried blood spots (DBS) with LC-MS/MS. Journal of Pharmaceutical and Biomedical Analysis. 2014;88:144–151. doi: 10.1016/j.jpba.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]