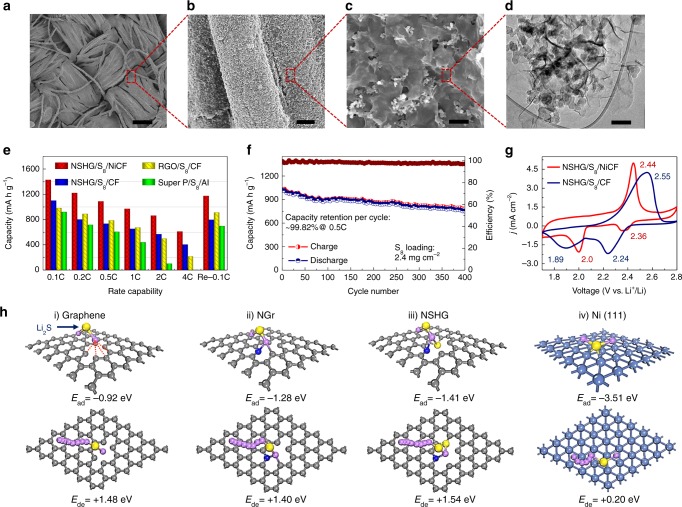
Fig. 4.
Cycling stability and rate capability of sulfur cathode. a–c Low and high magnification scanning electron microscopy (SEM) images of the NSHG/S8/Ni cathode (NSHG is nitrogen and sulfur heavily doped graphene). The sulfur cathode was obtained by direct infiltration of NSHG/S8 hybrid inks into the nickel-deposited carbon fabric (NiCF), followed by drying at 60 °C in a vacuum chamber. Scale bar for a–c is 100 µm, 5 µm, and 500 nm, respectively. d Transmission electron microscopy (TEM) images of NSHG/S8 cladding layers, extracted from NSHG/S8/Ni cathode (scale bar = 200 nm). e Specific capacities at various rates for sulfur cathodes, including NSHG/S8/NiCF, NSHG/S8/CF (CF is carbon fabric), RGO/S8/NiCF (RGO is reduced graphene oxide) and Super P/S8/Al electrodes (Super P is a type of carbon black). f Specific capacities, and corresponding CE values of the optimized NSHG/S8/NiCF cathode were obtained at 2 mA cm−2 for 400 cycles. g Cyclic voltammetry curves of NSHG/S8/NiCF and NSHG/S8/CF at a scan rate of 0.2 mV s−1 in a potential window from 1.7 to 2.8 V. h Theoretical calculation of the adsorption and decomposition energies of Li2S on the surface of RGO, nitrogen-doped graphene (NGr), NSHG, and the thin slab of Ni (111). Notably, the thin slab of Ni (111) with a large adsorption energy of −3.51 eV and a tiny decomposition energy barrier of 0.2 eV for Li2S facilitates the rapid oxidation of Li2S back to sulfur (carbon is gray, sulfur is yellow, lithium is purple, nitrogen is blue, nickel is light blue)