Fig. 4.
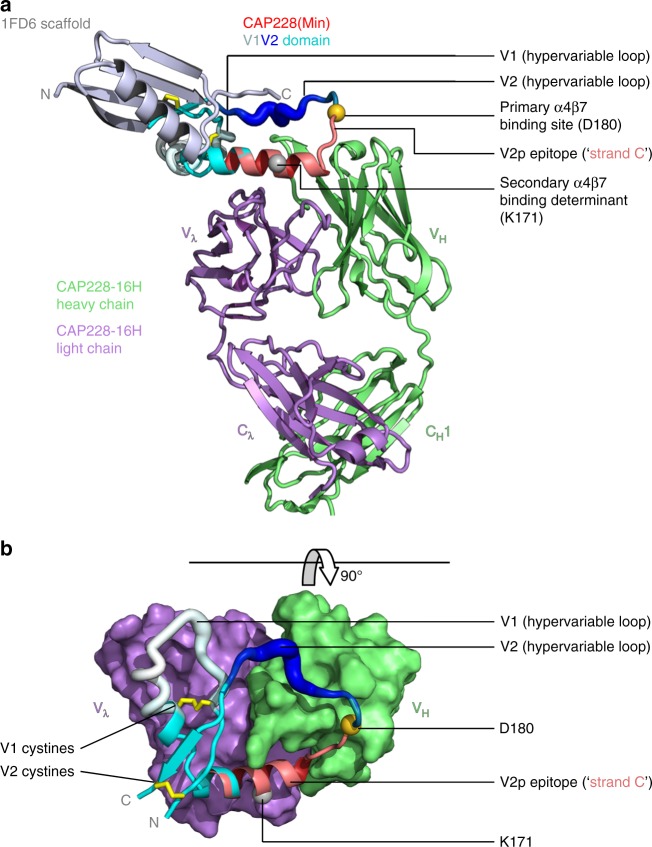
Cocrystal structure of a scaffolded, helical V1V2 domain bound to CAP228-16H. a The 1FD6 scaffold (metallic blue), autologous CAP228(Min) V1V2 domain (multicolour) and CAP228-16H Fab heavy and light chains (green and purple, respectively) are shown in cartoon view. V1V2 regions are labelled as hypervariable loop 1 (residues 132–156, white), hypervariable loop 2 (residues 183–196, dark blue), and C strand (residues 167–179, red), with the remainder coloured cyan. Internal V1V2 disulphide bonds are shown as yellow sticks, while the primary α4β7 binding site and potential secondary α4β7 binding determinant are indicated with the gold and silver spheres, respectively. b The view has been rotated 90°, and the 1FD6 scaffold (which would point ‘out of the page’) has been omitted to clearly show the interaction. The CAP228-16H paratope is shown in surface view, with the V1V2 domain still in cartoon representation, coloured as above