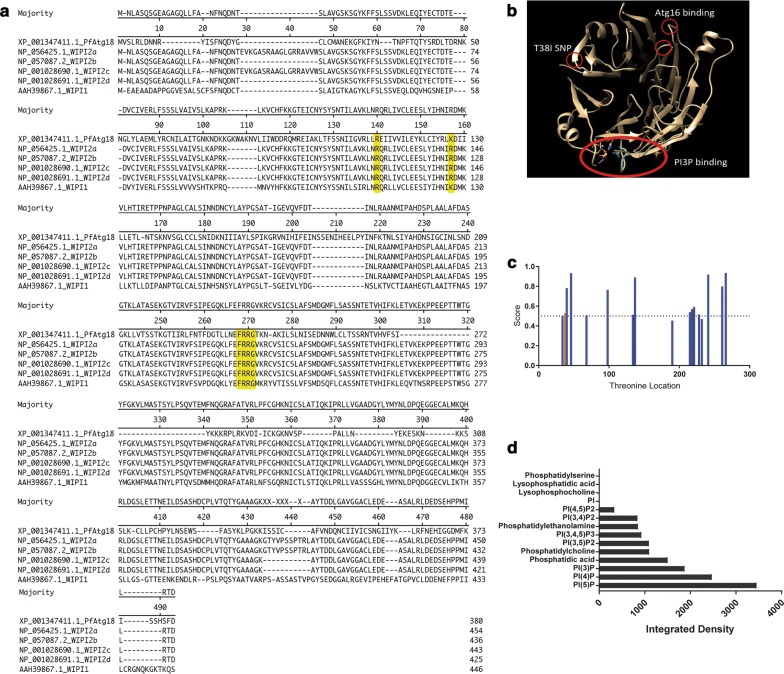
Fig. 1.
PfAtg18 is capable of binding PI3P. a The alignment of the protein sequence of P. falciparum PF3D7_1012900, now annotated as autophagy-related gene 18 (atg18), is similar to mammalian homologues, the WD-repeat protein Interacting with Phosphoinositides (WIPI) proteins. Conserved binding domains are highlighted in yellow. WIPI protein FRRG domain binds PI3P while the double arginine residues bind Atg16. b Image depicts P. falciparum Atg18 protein modeled based on homologous Hsv2 protein in yeast. Putative binding sites for PI3P and Atg16 are highlighted, as well as the location of the T38I SNP (red circles). c The threonine amino acids in PfAtg18 are shown with their likelihood of being phosphorylation sites. The T38 site is shown in orange. The threshold (dotted line) is 0.5. d Binding of Atg18 from Dd2R539T GFP-tagged Atg18 parasite line to various lipids based on integrated density from an Echelon PIP strip membrane demonstrates binding to PI3P