Table 2.
Drugs under Clinical Development.
| Drug | Chemical Class | Phase | Mechanism of Action |
|---|---|---|---|
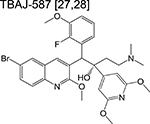 |
diarylquinoline | Pre-phase 1 | Inhibits ATP synthase and inhibits respiration |
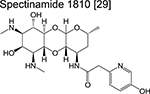 |
spectinamide | Pre-phase 1 | Binds to the ribosome and inhibits protein synthesis |
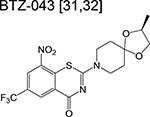 |
benzothiazinone | 1 | Forms a covalent adduct with DprE1 and inhibits arabinogalactan synthesis |
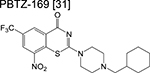 |
benzothiazinone | 1 | Forms a covalent adduct with DprE1 and inhibits arabinogalactan synthesis |
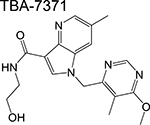 |
azaindole | 1 | Binds to DprE1 and inhibits arabinogalactan synthesis |
| OPC-167832* | dihydrocarbostyril | 1 | Binds to DprE1 and inhibits |
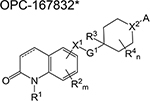 |
arabinogalactan synthesis | ||
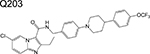 |
imidazopyridine | 1/2 | Binds to the QcrB subunit of cytochrome bc1 and inhibits respiration |
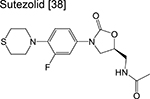 |
oxazolidinone | 1/2 | Binds to the ribosome and inhibits protein synthesis |
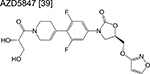 |
oxazolidinone | 2 | Binds to the ribosome and inhibits protein synthesis |
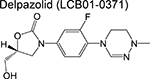 |
oxazolidinone | 2 | Binds to the ribosome and inhibits protein synthesis |
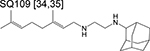 |
ethylenediamine | 2 | Binds to MmpL3 and inhibits cell wall synthesis |
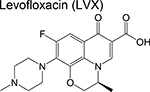 |
fluoroquinolone | 2 | Binds to DNA gyrase and inhibits DNA replication |
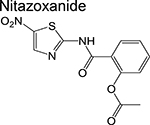 |
nitrothiazole | 2 | Disrupts the membrane potential and pH homeostasis |
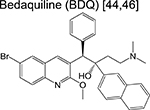 |
diarylquinoline | 3 | Binds to ATP synthase and inhibits respiration |
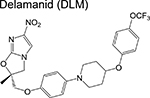 |
nitroimidazole | 3 | Blocks synthesis of mycolic acids and forms NO |
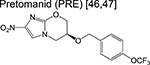 |
nitroimidazole | 3 | Blocks synthesis of mycolic acids and forms NO. |
 |
riminophenazine | 3 | Reduced by NADH dehydrogenase II and subsequently forms reactive oxygen species |
The general structure of OPC-167832 shown here was taken from the US patent No. US2017/0253576 A1.
More compounds in clinical development can be found at www.newtbdrugs.org
