Figure 1.
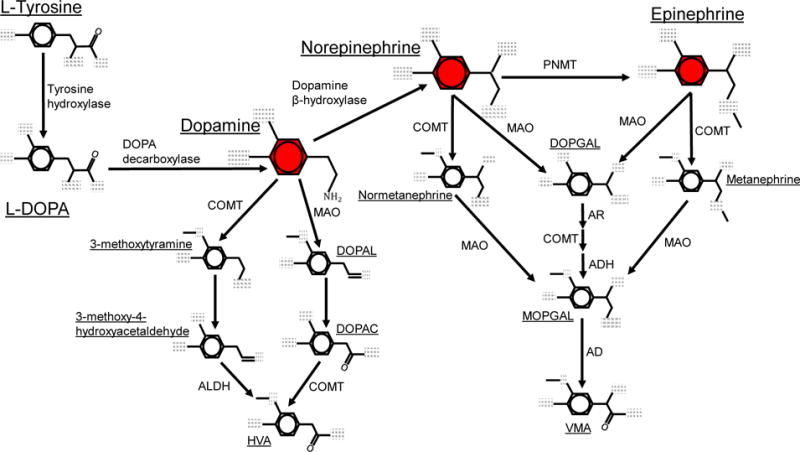
Metabolic pathways for catecholamine biosynthesis and degradation. Metabolic pathways for the formation and degradation of dopamine, norepinephrine and epinephrine (shown in red) are described. Catecholamine synthesis is initiated with the hydroxylation of the amino acid tyrosine is by tyrosine hydroxylase (TH), generating L-DOPA. L-DOPA is then converted to dopamine by DOPA decarboxylase (DDC, also known as aromatic L-amino acid decarboxylase, AADC). Dopamine is hydroxylated by dopamine β-hydroxylase (DBH) to form norepinephrine, which is then converted to epinephrine by phenylethanolamine-N-methyltransferase (PNMT). The catecholamines are primarily metabolized by two enzymatic pathways with catechol-O-methyltransferase (COMT) and monoamine oxidase (MAO). COMT converts dopamine to 3-methoxytyramine, norepinephrine to normetanephrine, and epinephrine to metanephrine via meta-O-methylation. MAO converts dopamine to 3,4-Dihydroxyphenylacetaldehyde (DOPAL), and norepinephrine or epinephrine to 3,4-didydroxyphenylclycoaldehyde (DOPGAL) by oxidative deamination. MAO also converts 3-methoxytyramine to 3-methoxy-4-hydroxyacetaldehyde, and the metanephrines to an unstable aldehyde monohydroxyphenylglycol aldehyde (MOPGAL). MOPGAL is ultimately converted to the final product vanillyl mandelic acid (VMA) by aldehyde reductase. In the final steps of dopamine metabolism, COMT and aldehyde dehydrogenase (ALDH) convert DOPAL and 3-methoxy-4-hydroxyacetaldehyde to the final product homovanilic acid (HVA).
