Abstract
OBJECTIVE
Our goal was to estimate the impact of a Shigella vaccine in an area where shigellosis is endemic by characterizing the disease burden and antibiotic-resistance profiles of isolates and by determining the prevalence of Shigella flexneri serotypes.
PATIENTS AND METHODS
We conducted a 43-month-long prospective, community-based diarrheal disease surveillance in 442 children <72 months of age in the Peruvian Amazon between October 1, 2002, and April 15, 2006.
RESULTS
The incidence of diarrheal disease was 4.38 episodes per child-year. The incidence rate for shigellosis was 0.34 episodes per child-year in children <72 months of age and peaked in children between 12 and 23 months at 0.43 episodes per child-year. Maternal education at or beyond the primary grade level, piped water supply, weight-for-age z score, and improved water-storage practices were the most significant determinants of disease in this community with living conditions comparable to many rural areas in the developing world.
CONCLUSIONS
Children living in this region had a 20-fold higher rate of disease incidence detected by active surveillance as those recently estimated by passive detection. Most symptomatic disease was caused by S flexneri, although the diversity of serotypes will require a multivalent vaccine to have a significant impact on the burden of disease caused by shigellosis. Several other public health disease-control interventions targeted at water source and improved storage, nutritional interventions, and improved maternal education seem to have a greater impact than a univalent S flexneri 2a vaccine.
Keywords: antibiotic resistance, bacterial infections, diarrhea, nutrition, vaccines
Diarrhea causes 1 billion illness episodes and 2.5 million deaths per year of children under the age of 5 in the developing world and China.1 Improved case management of acute dehydrating diarrhea through the use of oral rehydration therapy2 has increased the relative importance of dysentery and persistent diarrhea as the cause of severe and fatal disease.3,4 Shigella is a major cause of diarrhea and mortality in the developing world5 and is the enteric infection that has been most consistently associated with the clinical dysentery syndrome,6,7 prolonged episodes of acute diarrhea, and the development of persistent diarrhea.8 Episodes of shigellosis may result in a protein-losing enteropathy9 and have been shown to be a risk factor for subsequent malnutrition and growth shortfalls.10 Antibiotic resistance is becoming a progressive problem in southeast Asia,11,12 Africa,13 and South America.14,15 For these reasons, the World Health Organization has targeted the development of a vaccine for Shigella as a high priority.
Three key factors are elemental in the adequate control of the morbidity and mortality resulting from shigellosis. The first factor is regular periodic antibiotic-susceptibility testing of isolates to guide local empiric therapy for dysentery. The second factor is intermittent regional monitoring of disease burden with serotype breakdown to guide vaccine development and implementation and identify sites for vaccine trials. The final factor is the determination of risk factors to guide disease-control interventions. We sought to measure the disease burden, the prevalence of antibiotic resistance in Shigella isolates, the distribution of serotypes, and risk factors for shigellosis in the context of rural South America.
METHODS
Site and Surveillance
Santa Clara is a rural community in the Peruvian Amazon located 15 km southeast from the urban center of Iquitos. The study cohort was chosen after a community census that generated a list of children <70 months of age. Enrollment was limited to 1 child per household. Selection included every third child on the census list sorted according to date of birth. Ninety-six percent of families invited to participate gave informed consent for enrollment in the study protocol. After 72 months of age, the child was removed from surveillance and the next live birth was enrolled. The study protocol was approved by the institutional review boards of Johns Hopkins Bloomberg School of Public Health (Baltimore, MD), US Navy Medical Research Center (Silver Springs, MD), Asociacion Benefica PRISMA (Lima, Peru), and the Regional Health Department of Loreto Peru.
Children were weighed on Salter scales (Salter Housewares Ltd, Tonbridge, England), and their height was measured by using a marked platform with a sliding footboard at 1-month intervals, with each child being measured on the day of their birth. Participating families were visited 3 times weekly by a trained health promoter to document the number, consistency, and characteristics of stool passed over the previous 24-hour period, as well as other gastrointestinal symptoms, fever, anorexia, and treatments given. Reference stool cultures were obtained quarterly to determine asymptomatic carriage rates of Shigella and other enteropathogens.
Diarrhea was defined as an overt change in a child’s normal stool pattern characterized by an increase in the frequency to at least 3 unformed stools in a 24-hour period. Dysentery was defined as a diarrheal episode in which a technician observed blood in an unformed stool that coincided with a diarrheal episode on active surveillance. Episodes of diarrhea and dysentery were considered to be separate if separated by 3 diarrhea-free days,16 and the episode was considered attributable to the enteric pathogen if the pathogen was isolated during or within 2 days of the episode. Shigellosis was defined as a diarrheal episode from which Shigella was isolated on stool culture.
Specimens were obtained by field workers as soon as possible after meeting the case definition for diarrhea. One sample was sought for all episodes; however, children who were culture-negative for Campylobacter and Shigella but continued to have diarrhea were asked to provide a sample every fourth day until the episode remitted.
Microbiology
Stool
Fresh voided stools were examined for the presence of blood, mucus, and the state of formation. Testing for occult blood was performed by using hemoccult (Smith-Kline Diagnostics, Palo Alto, CA) cards, and a fecal smear was stained with methylene blue to measure intestinal inflammation. An aliquot was placed in Cary-Blair medium, refrigerated, transported in a cooler from the field site, and plated on the day of collection. Samples were directly plated on MacConkey, Hektoen, and Blaser’s medium. Samples were plated on thiosulfate-citrate bile salts after 6 to 8 hours of pre-enrichment in alkaline peptone water. Colonies displaying an appearance consistent with Shigella by standard biochemical testing were confirmed by agglutination with serogroup specific antisera (Denka-Seiken, Tokyo, Japan). Shigella flexneri isolates were further typed by agglutination with type-specific antisera (Denka-Seiken). Antibiotic sensitivity was tested by using the disk-diffusion method.
Polymerase Chain Reaction
Selected stools frozen at −20°C were processed for invasion plasmid antigen H (IpaH) as per previously described methods.17,18 Polymerase chain reaction (PCR) was run with an initial 5 minutes at 94°C followed by 35 cycles of 94°C for 30 seconds, 55°C for 1 minute, and 72°C for 1 minute, and a final 5 minutes at 72°C. PCR products were electrophoresed on a 2% agarose gel and stained with ethidium bromide and visualized with UV light.
Risk Factors
Risk factors examined in the analysis included demographic and socioeconomic factors, the source and quantity of water available, the use of latrines and the presence of drainage ditches/open sewers in and around the household, and nutritional status. All data except those related to nutritional status and breastfeeding data were derived from the census. Demographic factors included age and gender of the child, maternal age and education (maximum grade level obtained), and people per area of roofed area (people per square meter). Socioeconomic indicators included family and per-capita monthly income, and proxy economic variables regarding the cost of the materials used for housing. Six water-use variables were assessed: (1) the source of water used for drinking (categorized as a piped chlorinated water supply to the household, piped chlorinated water available from a public tap, a deep sealed well, shallow improvised wells, and river/inlet); (2) the volume of the smallest container used for water storage; (3) the number of hours in the day in which piped water was available; (4) total volume of water stored per capita; (5) per-capita volume of water stored with properly sealing tops; and (6) distance from the water source to the kitchen. For sanitation, latrine presence and sharing of a latrine (with >1 household) were recorded. Additional sanitation variables examined included the presence of drainage ditches/open sewers in and around the household and whether they were covered or were reported to overflow into the living area of the family. Nutritional factors examined included the birth weight and the height-forage z score (HAZ), weight-for-age z score (WAZ), and weight-for-height z score (WHZ). The relationship of breastfeeding to disease incidence was determined by classifying time periods according to months of exclusive breastfeeding (defined as breast milk only with no additional intake of liquids and solids other than medicines), partial breastfeeding (breastfeeding with additional liquids given), mixed breastfeeding (breastfeeding with additional liquids and solids), and weaned.
Analysis
Raw incidence rates (IRs) for diarrheal illness, shigellosis, and dysentery were calculated by dividing the number of incident episodes by the observed time at risk. Generalized estimating equations (GEEs) were used to estimate robust 95% confidence intervals (CIs) for IRs and to conduct risk-factor analysis.19,20 Ninety-five percent CIs were computed by modeling the number of incident episodes per child-year in the study, whereas risk factor analysis modeled the odds of experiencing an episode of Shigella given a hypothesized risk factor.
The multivariate model used GEEs to estimate a logistic regression to model the probability of shigellosis. The model was selected by identifying a maximum set of variables on the basis of examination of descriptive results. Variables were excluded in a stepwise fashion if P values from Wald statistics were >.40 and if the quasi-likelihood information criterion (QIC) statistic indicated improved model fit on variable exclusion.21 Variables examined descriptively that did not enter the maximum model were evaluated for entry after a final full model was chosen. Because of curvilinear relationships, higher power terms for age, normalized anthropometric measures, and time (as continuous variables) were explored and compared with categorical definitions of these variables. The QIC statistic was used to evaluate fit between categorical and continuous definitions. In addition to QIC, goodness of model fit was evaluated by using Horton et al’s goodness-of-fit test for logistic regression estimated by GEE.22 Odds ratios are reported, which we interpret in terms of relative risk given our large sample and satisfaction of the rare outcome assumption. Anthropometric data were converted to z scores by using the World Health Organization standards from 1978. Statistical analyses were performed by using SAS 9.2 (SAS Institute, Inc, Cary, NC).
RESULTS
Community Sociodemographic Profile
The community census identified 3472 individuals in 612 households over the study period. The mean monthly per-capita income was $28 US dollars. Sixty-one percent of households obtained water from a municipal system of water delivering chlorinated water to individual households from between 2 to 4 hours a day. The community lacked a centralized sewage system; however, 82.9% of the families used latrines as opposed to open ditches or other open areas for excrementation. Mothers of study children were well educated. Less than 1% was illiterate, and 48% had advanced past primary school level.
Four hundred forty-two children were enrolled in the study and contributed to a total of 914.3 child-years of surveillance. The number of child-years of surveillance is less than the product of participants and the study period because of staggered enrollment, exclusion on the completion of 72 months of life, and because of temporary intermittent out-migrations of the study children from the surveillance area. One child was withdrawn by the mother, and 3 children were lost to external migration. There were no deaths in the cohort during the study period.
Incidence of Diarrhea and Dysentery
In the 334 030 child-days of surveillance, there were 4023 episodes of diarrhea detected for an overall incidence of 4.38 episodes per child-year (95% CI: 4.09–4.68). The mean duration of observed episodes was 3.6 days, and the study population spent 4.2% of the total surveillance period with diarrhea. Only 1.7% of episodes persisted beyond 14 days. Of the total number of episodes of diarrhea, samples were available for etiologic analysis in 3756 episodes (93% of all observed episodes). Ninety percent (89.6%) of the study children experienced diarrhea during the period of their active surveillance. Table 1 shows the incidence of all-cause diarrhea, dysentery, shigellosis, and S flexneri according to age group.
TABLE 1.
IRs of Diarrheal Disease, Dysentery, Shigellosis, and Shigellosis Caused by S flexneri
| Age, mo | Child-Years | Diarrhea, IR (95% CI) | Dysentery, IR (95% CI) | Shigella (All), IR (95% CI) | S flexneri, IR (95% CI) |
|---|---|---|---|---|---|
| 0–5 | 47.1 | 6.84 (6.07–7.43) | 0.06 (0.02–0.19) | 0.23 (0.13–0.46) | 0.17 (0.08–0.37) |
| 6–11 | 62.4 | 7.21 (6.55–7.74) | 0.19 (0.10–0.36) | 0.16 (0.08–0.31) | 0.08 (0.03–0.22) |
| 12–23 | 150.3 | 7.29 (6.77–7.66) | 0.36 (0.27–0.47) | 0.43 (0.34–0.53) | 0.30 (0.23–0.39) |
| 24–35 | 161.3 | 4.99 (4.57–5.45) | 0.34 (0.25–0.45) | 0.40 (0.31–0.51) | 0.27 (0.20–0.35) |
| 36–47 | 165.4 | 3.52 (3.16–3.97) | 0.16 (0.11–0.23) | 0.39 (0.30–0.51) | 0.26 (0.19–0.36) |
| 48–59 | 172.5 | 2.59 (2.30–2.95) | 0.12 (0.08–0.18) | 0.38 (0.29–0.48) | 0.25 (0.19–0.34) |
| 60–72 | 155.5 | 2.08 (1.74–2.48) | 0.07 (0.04–0.13) | 0.21 (0.14–0.30) | 0.14 (0.09–0.21) |
| All | 914.4 | 4.40 (4.09–4.68) | 0.20 (0.17–0.23) | 0.34 (0.30–0.38) | 0.23 (0.20–0.26) |
IRs are reported as raw rates per child-year. The 95% CIs are computed by using GEE.
One hundred eighty-one episodes of diarrhea (4.4%) were dysenteric. The peak incidence of dysentery was 0.36 episodes per year (95% CI: 0.27–0.47) in children between 12 and 23 months of age. The overall incidence of dysentery using this definition in children in the cohort was 0.20 (95% CI: 0.17–0.23). Shigellosis was the most common bacterial isolate in cases of dysentery, and 34.8% of dysenteric episodes were attributable to Shigella, with an additional 16% attributable to Campylobacter. PCR performed on all available dysenteric specimens (139, representing 78% of all dysenteric episodes) revealed that 64% tested positive for Shigella. These were the only enteropathogens found to be associated with dysentery when compared with watery diarrhea.
Incidence and Clinical and Laboratory Characteristics of Shigellosis
Shigella was detected in 8.3% of all diarrheal episodes for which stool specimens were available and had an annual incidence of 0.34 episodes per child-year (95% CI: 0.30–0.38). Shigella incidence was lowest among 0- to 5-month-olds and 6- to 11-month-olds at 0.23 and 0.16 episodes per child-year, respectively. Shigellosis resulted in overt dysentery in only 20.2%, whereas the remainder of cases appeared as acute watery diarrhea. Shigellosis was associated with fecal occult blood in 30.8% of documented episodes and fecal leukocyte detection in 23.4% of episodes, significantly more than in all-cause diarrhea (7.9% and 10.9%; P < .001). The mean duration of an episode of shigellosis was 3.68 days (range: 1–31 days). Shigellosis presented with fever in 73.4% of all episodes and was more likely to be associated with fever than all-cause diarrhea (58.6%; P < .001).
Shigella isolates were also obtained from 3.2% of surveillance stool cultures from children in the absence of diarrheal illness. One hundred three isolates of the 414 isolates analyzed were from asymptomatic children. S flexneri accounted for 67.1% (278) of isolates, Shigella sonnei for 11.8% (49), Shigella boydii for 11.4% (47), Shigella dysenteriae for 2.4%, and other Shigella species that exhibited a biochemical profile of Shigella but did not agglutinate with type specific sera, 7.2% (30). Serotyping of S flexneri isolates revealed 33.1% of all S flexneri and 1.8% of all diarrheal episodes at the community level were caused by S flexneri 2a. S flexneri serotype 3a was present in 19.4%, S flexneri serotype 6 was present in 16.5%, and S flexneri 4a was present in 10.1% of all flexneri isolates. The remaining S flexneri serotypes accounted for <10% of S flexneri isolates and are listed in Table 2.
TABLE 2.
Distribution of S flexneri Serotypes in Asymptomatic and Symptomatic Children
| Serotype | No. of Isolates (% of S flexneri Isolates) in Symptomatic Children |
No. of Isolates (% of S flexneri Isolates) in Asymptomatic Children |
No. of isolates (% of S flexneri Isolates) in Study Cohort |
|---|---|---|---|
| 1a | 2 (1.0) | 0 (0.0) | 2 (0.7) |
| 1b | 18 (8.7) | 9 (12.9) | 27 (9.7) |
| 2a | 71 (34.1) | 21 (30.0) | 92 (33.1) |
| 2b | 2 (1.0) | 0 (0.0) | 2 (0.7) |
| 3a | 45 (21.6) | 9 (12.9) | 54 (19.4) |
| 3b | 2 (1.0) | 1 (1.4) | 3 (1.2) |
| 4a | 15 (7.2) | 13 (18.6) | 28 (10.1) |
| 4b | 6 (2.9) | 4 (5.7) | 10 (3.6) |
| 6 | 35 (16.9) | 11 (31.4) | 46 (16.5) |
| X | 9 (4.3) | 1 (1.4) | 10 (3.6) |
| Y | 3 (1.4) | 1 (1.4) | 4 (1.4) |
| Total | 208 | 70 | 278 (67.1) |
Antibiotic Susceptibility Testing
Antibiotic testing was completed in 403 (97.3%) of 414 Shigella isolates from the study cohort. Overall isolates were highly resistant to designated first-line therapy. Seventy-three percent of isolates were resistant to ampicillin, 62% were resistant to chloramphenicol, 69% were resistant to erythromycin, 79% were resistant to trimethoprim-sulfamethoxazole, and 83% were resistant to tetracycline. Isolates showed increased susceptibility to ceftriaxone, azithromycin, nalidixic acid, and ciprofloxacin. Ninety-seven percent of the isolates were sensitive to ceftriaxone. In the case of azithromycin, 84% of the isolates were found to be sensitive, 11% intermediate, and 5% resistant, respectively. Ninety-five percent of isolates were sensitive to nalidixic acid, whereas 3% demonstrated intermediate susceptibility, and 2% of isolates were resistant. Results were similar for ciprofloxacin, as 97% of isolates were sensitive, 3% were intermediate, and 1% of isolates were resistant.
Risk-Factor Analysis
Univariate Analysis
Variation in risk across age categories is shown in Table 1. No differences in incidence of shigellosis were noted according to the gender of the child (P = .58), the age of the child’s mother (P = .25), or the presence of a younger child born within 2 years of the sentinel child in the household (P = .49) (see Table 3). Children whose mother’s received primary school or less education (<6 years) had a 52% higher (IR: 0.41 [95% CI: 0.36–0.48]) IR than those with more education (IR: 0.27 [95% CI: 0.22–0.32]; P < .005). Greater household crowding trended toward a higher shigellosis incidence, but the differences were not significant (P = .7). Lower monthly per-capita income and poor material used for flooring in the household were both correlated with shigellosis, and children living in houses with wood or bark floors had almost twice the rate of shigellosis as children living on cement floors (P = .01 and < .005, respectively).
TABLE 3.
Univariate Risk-Factor Analysis for Shigellosis
| Risk Factor | IR | 95% CI |
P |
|
|---|---|---|---|---|
| Overall | vs Referencea | |||
| Child’s gender | ||||
| Female | 0.35 | 0.29–0.41 | .5849 | Same |
| Male | 0.33 | 0.28–0.38 | ||
| Maternal age, y | ||||
| ≤19 | 0.27 | 0.19–0.38 | .2549 | .1123 |
| 20–24 | 0.39 | 0.31–0.47 | .7719 | |
| 25–29 | 0.33 | 0.25–0.41 | .5724 | |
| 30–34 | 0.32 | 0.24–0.41 | .4574 | |
| ≥35 | 0.37 | 0.28–0.47 | Reference | |
| Child <2 y younger than sentinel in household | ||||
| 0 | ||||
| ≥1 | ||||
| Maternal education | ||||
| Incomplete primary (<6 y) | 0.35 | 0.31–0.40 | .4928 | Same |
| Primary school or greater | 0.32 | 0.24–0.41 | ||
| Crowding: people per m2 in home | ||||
| Minimum–Q25 (0.00 to 0.08) | 0.31 | 0.24–0.40 | .7015 | .4342 |
| Q25–Q75 (0.08 to 0.18) | 0.35 | 0.29–0.41 | .8705 | |
| Q75–maximum (0.18 to 2.25) | 0.36 | 0.29–0.44 | Reference | |
| Monthly income per capitab | ||||
| Minimum–Q25 (0 to 35) | 0.42 | 0.35–0.52 | .0135 | Reference |
| Q25–Q75 (36 to 100) | 0.34 | 0.29–0.40 | .1094 | |
| Q75–maximum (101 to 350) | 0.26 | 0.20–0.34 | .0031 | |
| Floor material | ||||
| Dirt | 0.37 | 0.32–0.42 | .0027 | .7627 |
| Cement | 0.23 | 0.17–0.30 | .0446 | |
| Wood, bark | 0.40 | 0.25–0.64 | Reference | |
| Drinking water source | ||||
| Piped water to household | 0.32 | 0.28–0.37 | .1602 | .0002 |
| Piped water from public tap | 0.29 | 0.20–0.42 | .0011 | |
| Well | 0.39 | 0.30–0.49 | .0096 | |
| River or inlet | 0.70 | 0.48–1.03 | Reference | |
| Volume of smallest water container, L | ||||
| 0 (no storage) | 0.37 | 0.21–0.66 | .9662 | .7479 |
| 1–5 | 0.36 | 0.28–0.47 | .6499 | |
| 6–19 | 0.34 | 0.24–0.48 | .9319 | |
| ≥20 | 0.34 | 0.29–0.39 | Reference | |
| Water storage per person, L | ||||
| No storage | 0.32 | 0.15–0.67 | .4281 | .9196 |
| Minimum–Q25 (0.8 to 9.5) | 0.41 | 0.27–0.38 | .1076 | |
| Q25–Q75 (10 to 25) | 0.33 | 0.32–0.50 | .6896 | |
| Q75-maximum (>25) | 0.31 | 0.24–0.38 | Reference | |
| Water storage (L) per person with sealed tops | ||||
| No sealed storage | 0.44 | 0.35–0.54 | .0872 | .0126 |
| Minimum–Q25 (<4) | 0.33 | 0.24–0.45 | .4987 | |
| Q25–Q75 (4.0 to 11.5) | 0.32 | 0.26–0.37 | .5723 | |
| Q75–maximum (≥12) | 0.29 | 0.21–0.37 | Reference | |
| Distance, water source to kitchen, m | ||||
| 0.0–1.5 | 0.33 | 0.26–0.41 | .1250 | .1373 |
| 2.0–3.5 | 0.28 | 0.23–0.35 | .0145 | |
| 4.0–9.5 | 0.37 | 0.29–0.47 | .3244 | |
| ≥ 10.0 | 0.41 | 0.33–0.51 | Reference | |
| Type of latrine | ||||
| Family bathroom (inside home) | 0.20 | 0.07–0.52 | .1247 | .2248 |
| Family latrine (outside home) | 0.36 | 0.31–0.40 | .9478 | |
| Shared latrine | 0.28 | 0.21–0.36 | .1279 | |
| Well, river, or field | 0.36 | 0.26–0.48 | Reference | |
| Sewers in/around household covered | ||||
| Yes | 0.22 | 0.14–0.34 | .0397 | Same |
| No | 0.36 | 0.32–0.40 | ||
| Birth weight | ||||
| <2.5 kg | 0.39 | 0.26–0.58 | .4864 | Same |
| ≥2.5 kg | 0.34 | 0.30–0.38 | ||
| Anthropometrics, HAZ | ||||
| Minimum–Q25 (−5.68 to 2.32) | 0.41 | 0.33–0.52 | .2462 | .0939 |
| Q25–Q75 (−2.32 to 1.14) | 0.32 | 0.27–0.38 | .7321 | |
| Q75–maximum (−1.1 to4.44) | 0.30 | 0.24–0.38 | Reference | |
| Anthropometrics, WAZ | ||||
| Minimum–Q25 (−3.91 to 1.38) | 0.39 | 0.30–0.49 | .0032 | .0033 |
| Q25–Q75 (−1.38 to 0.16) | 0.37 | 0.31–0.43 | .0062 | |
| Q75–maximum (−0.16 to4.77) | 0.22 | 0.17–0.29 | Reference | |
| Anthropometrics, WHZ | ||||
| Minimum–Q25 (−3.18 to 0.1) | 0.37 | 0.29–0.46 | .0050 | .0098 |
| Q25–Q75 (−0.1 to 0.91) | 0.38 | 0.32–0.44 | .0045 | |
| Q75–maximum (0.91 to 5.78) | 0.23 | 0.18–0.30 | Reference | |
| Breastfeeding Status (children enrolled at <9mo age) | ||||
| Exclusive | 0.16 | 0.06–0.41 | .1270 | .1131 |
| Mostly exclusive | 0.16 | 0.03–0.89 | .3914 | |
| Partial breastfeeding | 0.31 | 0.21–0.45 | .5281 | |
| Weaned | ||||
| Breastfeeding status (all children) | ||||
| Exclusive | 0.35 | 0.28–0.43 | Reference | |
| Mostly exclusive | 0.25 | 0.14–0.49 | .4728 | .3876 |
| Partial breastfeeding | 0.16 | 0.03–0.89 | .3997 | |
| Weaned | 0.35 | 0.25–0.48 | .9620 | |
| Exclusive | 0.35 | 0.30–0.39 | Reference | |
| Season | ||||
| November–March, all years | 0.21 | 0.16–0.26 | <0001 | Reference |
| March–October, 2003 | 0.56 | 0.46–0.68 | <.0001 | |
| March–October, 2004 | 0.44 | 0.34–0.55 | <.0001 | |
| March–October, 2005 | 0.29 | 0.22–0.38 | .0783 | |
| March–October, all years | 0.46 | 0.40–0.53 | <.0001 | |
Overall P values comparing >2 groups reflect differences among any of the groups. P values “vs Reference” reflects the comparison of each group (row) relative to the group (row) labeled as the reference group.
In Peruvian nuevo soles, 1 US dollars = 3.5 Peruvian soles at the time of the study.
The evaluation of drinking-water source revealed that children from households that used the river as the source of their drinking water had IRs >2 times that of piped water sources (IRR: 2.3; P < .001) and almost twice that of households who obtained water from wells (IRR: 1.8; P < .005). However, when improved water sources were compared, no difference was observed in IRs among children whose primary source of drinking water was a well, a connection to the municipal water supply that reached the household, or a connection to the municipal water supply that was available on the street. There was an increasing trend between the distance of water source from the kitchen to the incidence of shigellosis, which failed to reach significance (P = .12). Water storage was nearly universal, with 96% of the homes reporting storing water in containers. Neither the volume of the smallest water container used for water storage nor the total volume of water stored per capita was associated with the incidence of shigellosis (P = .97 and .43, respectively). However, the storage of water in containers with properly sealing lids (plastic lids on buckets that were properly sized for the container) was highly protective; children living in these homes had 32.8% decrease in the incidence of shigellosis compared with children living in homes storing water in open containers or containers sealed with poorly fitting lids or cloth (IR: 0.31 vs 0.46; P = .04). The presence of a latrine was not associated with risk for shigellosis. However, sewers in and directly surrounding the household (present in 93.6% of study households) are common in this community with torrential rains and yearly rainfall of >3 m. The presence of improvised covers on sewers running in and around the household was associated with a 39% reduction in incidence (P = .04) compared with houses with uncovered sewers.
There was no increased incidence of shigellosis in low birth weight children. The HAZ exhibited a negative trend in respect to the risk of developing shigellosis; however, this trend did not reach statistical significance (P = .25). However, WAZ and WHZ scores were negatively correlated with the incidence of shigellosis. Children in the lowest WAZ quartile had a 77% increase in shigellosis incidence relative to children in the highest WAZ quartile (P = .003), and children in the lowest WHZ quartile had a 61% increase in incidence relative to children in the highest WHZ quartile (P = .01). For children who entered the study under the age of 9 months, breastfeeding status was associated with a 54.3% decrease in the shigellosis incidence in exclusively breastfed children relative to that of weaned children (P = .11) that was not statistically significant. When all children were considered independent of their time of enrollment, breastfeeding was not significantly correlated with the development of shigellosis despite a trend toward a decrease in incidence in exclusively or predominantly breastfed children.
The incidence of disease was evaluated temporally according to season. Transmission intensity was greater in the months of March to October than in November to February. The IRs in the high-transmission seasons were 2.23 times that of the low-transmission seasons (P < .0001). The incidence of shigellosis during low-transmission seasons was 0.21 episodes per child-year (95% CI: 0.16–0.26) and was not significantly different between years in the 3-year study period. The incidence during peak transmission seasons in 2003 (IR: 0.56 [95% CI: 0.46–0.68]) and 2004 (IR: 0.44 [95% CI: 0.34–0.55]) were not significantly different from each other (P = .10) but were significantly higher than the peak in 2005 (IR: 0.29 [95% CI: 0.22–0.38]), largely because of an early decrease in cases in September and October 2005. If the high-transmission season in 2005 was truncated to March through August, the IRs during this season increase to 0.36 episodes per child-year (95% CI: 0.27–0.47), slightly lower than in the 2 previous seasons (P = .04).
Multivariate Model
Descriptive results identified the following maximum set of variables for model selection: age of the child, maternal education, per-capita income, materials used for flooring, water source, sealed water storage, latrine type, coverage of sewers around household, anthropometrics (added separately), breastfeeding status, and seasonality. Model selection procedures resulted in the selection of the subset of variables seen in Table 4. The final model included linear, quadratic, and cubic terms for child’s age and WAZ; however, a categorical definition for time defined as March through October of each year was used to capture the seasonal effect. Child’s age was centered at 24 months and divided by 12 to ease interpretation of estimates (ie, age = [child’s age in months – 12]/24). The final model has a QIC of 2507.62 and Horton’s χ2 statistic of 8.34 (P = .50), both indicating good fit of the data.
TABLE 4.
Multivariate Model of Risk Factors for Shigellosis
| Variable | Odds Ratio | 95% CI | P |
|---|---|---|---|
| Agea | |||
| Linear | 1.30 | 1.11–1.52 | <005 |
| Quadratic | 0.78 | 0.67–0.92 | <005 |
| Cubic | 1.04 | 1.00–1.08 | .10 |
| Maternal education | |||
| ≥6 y vs >6y | 1.34 | 1.03–1.75 | .03 |
| Floor materialb | |||
| Dirt | 1.38 | 1.00–1.91 | .05 |
| Wood/bark | 1.30 | 0.79–2.15 | .30 |
| Cement | Ref | — | — |
| Water sourcec | |||
| Community faucet | 0.44 | 0.25–0.76 | <005 |
| Household faucet | 0.52 | 0.34–0.80 | .003 |
| Well | 0.50 | 0.31–0.82 | .006 |
| River | Ref | — | — |
| Sewers in/around house | |||
| Covered vs open | 0.85 | 0.52–1.39 | .52 |
| Water storage | |||
| Sealed containers vs unsealed (or no storage) | 1.33 | 1.01–1.74 | .04 |
| WAZ | |||
| Linear | 0.70 | 0.52–0.93 | .02 |
| Quadratic | 0.92 | 0.84–1.01 | .08 |
| Cubic | 1.03 | 1.00–1.07 | .04 |
| Season | |||
| High vs low transmission | 2.07 | 1.55–2.78 | <005 |
Ref indicates reference group
In years, centered at 2 years (24 months)
Wald statistic: P = .14.
Wald statistic: P = .01
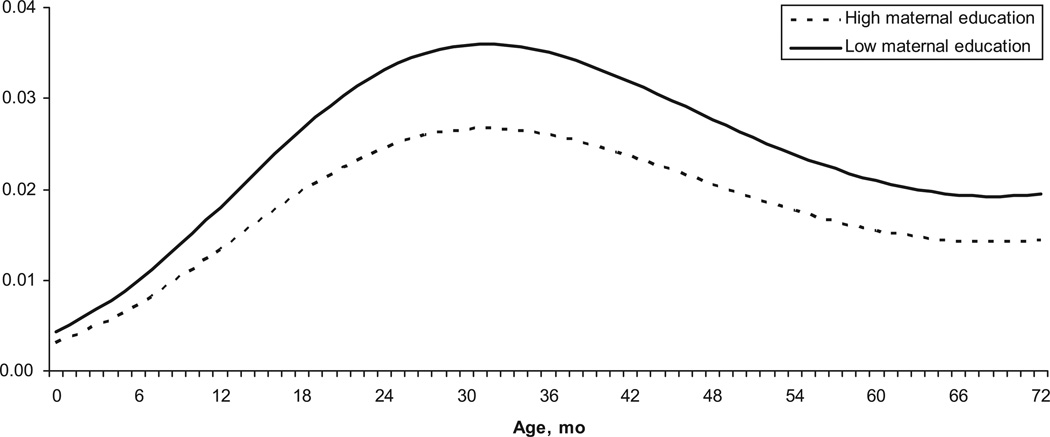
To interpret results, the reference group is described as a child 24 months of age with a WAZ of 0, maternal education of ≥6 years, in low-transmission season, who lives in a home with cement floors, uncovered sewers, uses the river as a drinking-water source, and stores water in a properly sealed container. There is a clear curvilinear relationship between child’s age and shigellosis risk. Children 12 months of age have approximately half the risk of shigellosis when compared with children over 18 months of age. Figure 1 shows the predicted risk of shigellosis for all ages in children with mothers who did not complete primary schooling versus those who did. Low maternal education is associated with a 34% increase in the odds of developing Shigella (P = .03).
FIGURE 1.
Predicted probability of shigellosis according to age and maternal education.
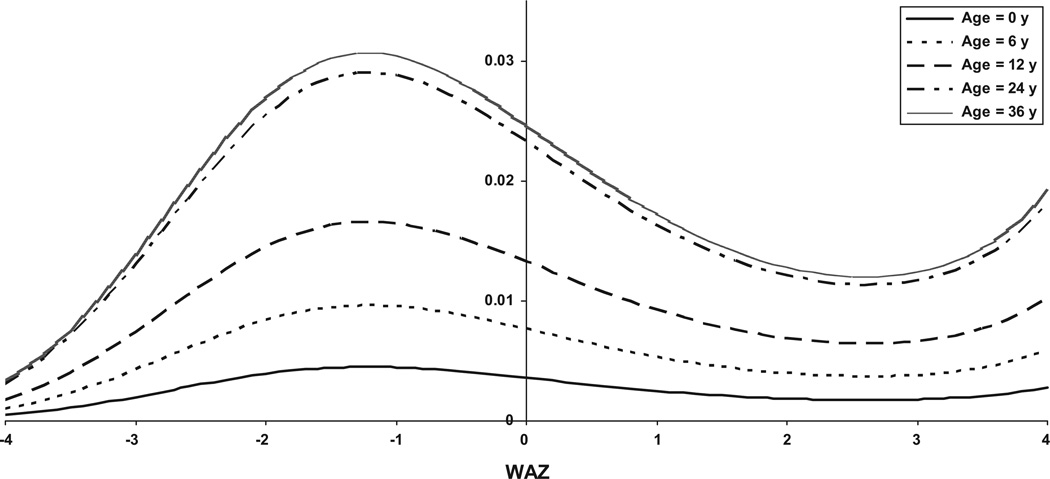
The effect of age is also apparent in examining the relationship between risk of shigellosis and underweight status (WAZ). The risk of shigellosis generally decreases with increasing WAZ, as seen in Fig 2; however, the effect of increasing WAZ on risk reduction varies according to age. Predicted probabilities from Fig 2 are based on model runs using different reference ages (ie, age = 0 corresponds to the same model in Table 4 that is centered on 0 and not 24 months, whereas age = 6 corresponds to centering on 6 months rather than 24). Being underweight clearly had a larger effect on the risk of older compared with younger children.
FIGURE 2.
Predicted probability (log odds) of shigellosis according to WAS for children at select ages.
Household socioeconomic status was captured by the materials used for the construction of floors. Having a dirt or wood/bark floor was associated with a 38% and 30% increase in Shigella risk compared with having a cement floor. Income per capita was considered as a replacement for floor material (results were similar in that lower income was associated with a higher risk of shigellosis); however, results were not significant and model fit did not improve when compared with the model with socioeconomic status expressed as material used for flooring.
Household handling of water and sewage also influenced shigellosis risk. Households with an improved water source (household faucet, community faucet, or well) had a 50% decreased risk of shigellosis compared with children living in households that obtained their drinking water from the river (P = .013). Households that did not store water in properly sealed containers had a 30% increased risk of disease (P = .04). The improvised covering of sewers was associated with a 15% reduction in risk, and although the relationship was not significant, the inclusion of this variable in the model improved the model fit according to the QIC statistic.
Seasonality was important in determining risk. Shigellosis risk more than doubled from March through October of each year compared with November through February (P < .0001), even after controlling for all other variables in the model.
DISCUSSION
The burden of diarrheal illness is high in this rural population in Peru. Ninety percent of children in the cohort had at least 1 diarrheal illness, and diarrhea was reported on 4.5% of the cohorts’ days under observation. IRs of diarrheal disease derived from active surveillance in a stable population in Santa Clara were relatively high compared with other rates in the literature published in the last 10 years, and age-adjusted rates were higher than current worldwide estimates of diarrheal incidence in under fives.23 The reported annual IRs of shigellosis are among the highest in the literature obtained at the community level. Black et al10 reported an isolation rate of 18.7% in Bangladesh in the 1970s, with a peak annual incidence of 1 episode per child-year in children 2 years of age, but since that time rates have fallen in Bangladesh.24 Other isolation and IRs for Shigella obtained by household surveillance in Chile (10%; 0.15 episodes per child-year),25 Guatemala (9.8%; 0.69 episodes per child-year),26 Egypt (3.6%; 0.2 episodes per child-year),27 and Thailand (4.9%; 0.04 episodes per child-year).28 In Chile, the peak incidence of Shigella infections of 0.17 infections per child-year occurred in children between 36 and 47 months of age. In Santa Clara, where disease transmission is more intense, the peak incidence of 0.43 infections per child-year was seen earlier in children 12 to 23 months of age, similar to the age peak in Egypt.27 Unlike Egypt, IRs for shigellosis were relatively stable over the 3-year surveillance period.
Recently, disease-burden estimates for shigellosis have been increasingly derived from passive detection.23,29,30 Although it is true that this allows for the inclusion of greater areas of study and is more economical, it is clear that significant underreporting occurs. A recent review from Vietnam, Bangladesh, Thailand, Pakistan, and Indonesia estimated an average of 0.013 episodes of shigellosis per child-year in children <5 years old, 20 times lower than seen under active surveillance in this study. Other similar passive surveillance systems have added correction factors (of 5 times the detected number of cases) to estimate true disease burdens.30 Because underreporting of disease is likely to be variable and depends on factors such as transport, health infrastructure, distance to health center, intensity of investigator presence, and local perceptions of disease severity, improved modeling of health care uses are needed before passive surveillance with estimated correction factors can replace community-based longitudinal studies to provide reliable estimates of disease burden.
Despite the fact that Shigella was the most common agent isolated in all children <5 years old with dysentery, the majority of episodes of shigellosis (79.8%) were nondysenteric. In the first year of life, rates of dysentery attributable to Campylobacter were nearly as frequent. Our rates of dysentery caused by Shigella are likely to be decreased by our interventions: all children were treated as soon as Shigella was isolated from a diarrheal stool and on the same day plating was done, which meant that antibiotics were generally being given on illness day 3. This likely prevented the evolution of more severe disease in this population. In addition, our definition of dysentery as visible blood in an unformed stool is more strict that that of other groups, which allow for microscopic criteria to be used.31
Although rates of diarrhea, dysentery, and shigellosis are high, it should be noted that the level of hygiene in this community is similar to that of many populations in the developing world. The community has completed all of the World Health Organization’s management of childhood illness guidelines for diarrhea. Standard case management is used and community knowledge and use of oral rehydration solutions are high. Vaccination coverage rates, including measles vaccination, surpass 95%. Furthermore, nearly all children receive near-exclusive breastfeeding until 6 months of age and are not completely weaned until 1 year of age, and an improved water source is available to >94.3% of the population. Despite these interventions, the burden of diarrheal illness, and shigellosis in particular, remain extremely high.
In this cohort, low maternal education, an untreated water source, poor water-storage practices, and being underweight were the key determinants of risk for shigellosis. Improved water storage in particular is an extremely affordable intervention that would seem to be an excellent disease-control strategy in this setting, because children living in homes where water was stored with perfectly fitted lids had a 31.8% decreased incidence of disease and local containers are available at a cost of $3 to $4 US dollars. Maternal education, targeted nutritional interventions to decrease the prevalence of underweight and wasted children, and improved water sources are other interventions that would be expected to have an important impact on the disease burden caused by shigellosis.
The Shigella isolated from children was resistant to the tetracycline, ampicillin, erythromycin, sulfamethoxazole/trimethoprim, and chloramphenicol, the treatments they were most likely to receive for dysentery, in >60% of all cases. Before the antibiotic-resistance data were available, the majority of children received ineffective drug therapy. Although resistance to ciprofloxacin, azithromycin, ceftriaxone, and nalidixic acid was uncommon, these medications are less available in this and other endemic areas. A policy change was necessary to make these antimicrobial agents available on an ongoing basis in this community, a change that has not yet been made on a regional or national level. Empiric strategies for the treatment of the dysentery syndrome need centrally planned periodic microbiologic evaluation to determine their adequacy. Current recommendations that more broadly specify an agent to which local strains are susceptible are not adequate given the lack of information regarding the susceptibility of isolates in most clinical contexts in endemic areas. Regional and national programs should recommend specific agents with microbiologic evidence of in vitro efficacy. Now that generic forms of ciprofloxacin and azithromycin are available and the cost of these more effective treatments has decreased significantly, policy makers should be able to recommend these more efficacious treatments with less reservation.
The use of a vaccine that offered complete protection against S flexneri 2a, 3a, and 6 would protect children against 72.6% of the incident cases of shigellosis caused by S flexneri but only 47.5% of all the cases of shigellosis. The most developed Shigella vaccines concentrate on S flexneri 2a alone, which in this population would have only a limited effect, because even a vaccine with 100% efficacy would prevent 34.1% of the cases of S flexneri but only 22.3% of the cases of Shigella and only 2% of all-cause diarrhea experienced by this cohort. In addition, because 24.2% of the episodes of shigellosis occurred in children under the age of 2 years, unless the vaccine is a conjugate or live-attenuated vaccine administered early in life, the efficacy is likely to be significantly hampered in this and other similar highly endemic settings.
What’s Known on This Subject.
Since the 1980’s the number of prospective cohort studies on diarrheal diseases has fallen markedly. Current disease burden estimates for shigellosis are based on active surveillance data that is often decades old or passive surveillance estimates.
What This Study Adds.
This study demonstrates high rates of shigellosis and brings into question the accuracy of recently used passive surveillance estimates, provides serotype-specific disease burdens to measure potential disease burden reduction achievable with Shigella vaccines, and compares this with alternative disease-control measures.
ACKNOWLEDGMENTS
This study was funded by National Institutes of Health grants K01-TW05717 (to Dr Kosek), U01 AI035894 (to Dr Gilman), and D43 TW006581 (to Dr Gilman). The work was further funded by Global Emerging Infections Surveillance and Response System work unit number 847705 82000 25GB B0016.
We thank M. A. Single for critical review of an earlier version of the manuscript and S. T. Unt for logistic and field support.
Abbreviations
- PCR
polymerase chain reaction
- HAZ
height-for-age z score
- WAZ
weight-for-age z score
- WHZ
weight-for-age z score
- GEE
generalized estimating equation
- CI
confidence interval
- QIC
Quasi-likelihood Information Criterion
- IR
incidence rate
- IRR
Incidence Rate Ratio
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or US government.
REFERENCES
- 1.Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81(3):197–204. [PMC free article] [PubMed] [Google Scholar]
- 2.Victora CG, Bryce J, Fontaine O, Monasch R. Reducing deaths from diarrhea through oral rehydration therapy. Bull World Health Organ. 2000;78(10):1246–1255. [PMC free article] [PubMed] [Google Scholar]
- 3.Victora CG, Huttly SR, Fuchs SC, Olinto MT. International differences in clinical patterns of diarrhoeal deaths: a comparison of children from Brazil, Senegal, Bangladesh, and India. J Diarrhoeal Dis Res. 1993;11(1):25–29. [PubMed] [Google Scholar]
- 4.Bhandari N, Bhan MK, Sazawal S. Mortality associated with acute watery diarrhea, dysentery and persistent diarrhea in rural north India. Acta Paediatr Suppl. 1992;381:3–6. [PubMed] [Google Scholar]
- 5.Kotloff KL, Winickoff JP, Ivanoff B, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77(8):651–666. [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor DN, Bodhidatta L, Echeverria P. Epidemiologic aspects of shigellosis and other causes of dysentery in Thailand. Rev Infect Dis. 1991;13(suppl 4):S226–S230. doi: 10.1093/clinids/13.supplement_4.s226. [DOI] [PubMed] [Google Scholar]
- 7.Taylor DN, Echeverria P, Pal T, et al. The role of Shigella spp., enteroinvasive Escherichia coli, and other enteropathogens as causes of childhood dysentery in Thailand. J Infect Dis. 1986;153(6):1132–1138. doi: 10.1093/infdis/153.6.1132. [DOI] [PubMed] [Google Scholar]
- 8.Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73(6):799–805. [PubMed] [Google Scholar]
- 9.Black RE, Levine MM. Intestinal protein loss in Shigellosis. Nutr Res. 1991;11(11):1215–1220. [Google Scholar]
- 10.Black RE, Brown KH, Becker S, Yunus M. Longitudinal studies of infectious diseases and physical growth of children in rural Bangladesh. Am J Epidemiol. 1982;115(3):315–324. doi: 10.1093/oxfordjournals.aje.a113307. [DOI] [PubMed] [Google Scholar]
- 11.Bennish ML, Salam MA, Hossein MA, et al. Antimicrobial resistance among Shigella isolates in Bangladesh, 1983–1990: increasing frequency of strains multiply resistant to ampicillin, trimethoprim-sulfamethoxazole and nalidixic acid. Clin Infect Dis. 1992;14(5):1055–1060. doi: 10.1093/clinids/14.5.1055. [DOI] [PubMed] [Google Scholar]
- 12.Dutta S, Chatterjee A, Dutta P, et al. Sensitivity and performance characteristics of a direct PCR with stool samples in comparison to conventional techniques for diagnosis of Shigella and enteroinvasive Escherichia coli infection in children with acute diarrhoea in Calcutta, India. J Med Microbiol. 2001;50(8):667–674. doi: 10.1099/0022-1317-50-8-667. [DOI] [PubMed] [Google Scholar]
- 13.Bogaerts J, Verhaegen J, Munyabikali JP, et al. Antimicrobial resistance and serotypes of Shigella isolates in Kigali, Rwanda (1983 to 1993): increasing frequency of multiple resistance. Diagn Microbiol Infect Dis. 1997;28(4):165–171. doi: 10.1016/s0732-8893(97)00072-2. [DOI] [PubMed] [Google Scholar]
- 14.Sua´rez ME, Carvajal L, Culasso C. Resistencia de Shigella spp a los antimicrobianos en Cordoba, Argentina, durante el periodo 1990–1997. Rev Panam Salud Publica. 2000;7(2):113–117. doi: 10.1590/s1020-49892000000200007. [DOI] [PubMed] [Google Scholar]
- 15.Lima AA, Lima NL, Pinho MC, et al. High frequency of strains multiply resistent to ampicillin, trimethoprim-sulfamethoxazole, streptomycin, chloramphenicol, and tetracycline isolated from patients with shigellosis in northeastern Brazil during the period 1988–1993. Antimicrob Agents Chemother. 1995;39(1):256–259. doi: 10.1128/aac.39.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baqui AH, Black RE, Yunus M, et al. Methodological issues in diarrhoeal diseases epidemiology: definition of diarrhoeal episodes. Int J Epidemiol. 1991;20(4):1057–1063. doi: 10.1093/ije/20.4.1057. [DOI] [PubMed] [Google Scholar]
- 17.Faruque SM, Khan R, Kamruzzaman M, et al. Isolation of Shigella dysenteriae type 1S flexneri strains from surface waters in Bangladesh: comparative molecular analysis of environmental Shigella isolates versus clinical strains. Appl Environ Microbiol. 2002;68(8):3908–3913. doi: 10.1128/AEM.68.8.3908-3913.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudio PA, Sethabutr O, Echeverria P, Hoge CW. Utility of a polymerase chain reaction diagnostic system in a study of the epidemiology of shigellosis among dysentery patients, family contacts, and well controls living in a shigellosis-endemic area. J Infect Dis. 1997;176(4):1013–1018. doi: 10.1086/516531. [DOI] [PubMed] [Google Scholar]
- 19.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 20.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]
- 21.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 22.Horton NJ, Bebchuk JD, Jones CL, et al. Goodness-of-fit for GEE: an example with mental health service utilization. Stat Med. 1999;18(2):213–222. doi: 10.1002/(sici)1097-0258(19990130)18:2<213::aid-sim999>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 23.Agtini MD, Soeharno R, Lesmana M, et al. The burden of diarrhoea, shigellosis, and cholera in North Jakarta, Indonesia: findings from 24 months surveillance [published correction appears in BMC Infect Dis. 2007;7:1] BMC Infect Dis. 2005;5:89. doi: 10.1186/1471-2334-5-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keusch GT, Fontaine O, Bhargava A, et al. Diarrheal Diseases. In: Jamison DT, Bremen JG, Mesham AR, et al., editors. Disease Control Priorities in Developing Countries. New York, NY: Oxford University Press; 2006. pp. 371–388. [Google Scholar]
- 25.Ferreccio C, Prado V, Ojeda A, et al. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in children in a poor periurban setting in Santiago, Chile. Am J Epidemiol. 1991;134(6):614–627. doi: 10.1093/oxfordjournals.aje.a116134. [DOI] [PubMed] [Google Scholar]
- 26.Ramiro Cruz J, Cano F, Bartlett AV, Mendez H. Infection, diarrhea, and dysentery caused by Shigella species and Campy-lobacter jejuni among Guatemalan rural children. Pediatr Infect Dis J. 1994;13(3):216–223. doi: 10.1097/00006454-199403000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Abu-Elyazeed RR, Wierzba TF, Frenck RW, et al. Epidemiology of Shigella-associated diarrhea in rural Egyptian children. Am J Trop Med Hyg. 2004;71(3):367–372. [PubMed] [Google Scholar]
- 28.Punyaratabandhu P, Vathanophas K, Varavithya W, et al. Childhood diarrhoea in a low-income urban community in Bangkok: incidence, clinical features, and child caretaker’s behaviours. J Diarrhoeal Dis Res. 1991;9(3):244–249. [PubMed] [Google Scholar]
- 29.von Seidlein L, Kim DR, Ali M, et al. A multicentre study of Shigella diarrhoea in 6 Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med. 2006;3(9):e353. doi: 10.1371/journal.pmed.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chompook P, Samosornsuk S, von Seidlein L, et al. Estimating the burden of shigellosis in Thailand: 36-month population-based surveillance study. Bull World Health Organ. 2005;83(10):739–746. [PMC free article] [PubMed] [Google Scholar]
- 31.Haque R, Mondal D, Kirkpatrick BD, et al. Epidemiologic and clinical characteristics of acute diarrhea with emphasis on Entamoeba histolytica infections in preschool children in an urban slum of Dhaka, Bangladesh. Am J Trop Med Hyg. 2003;69(4):398–405. [PubMed] [Google Scholar]