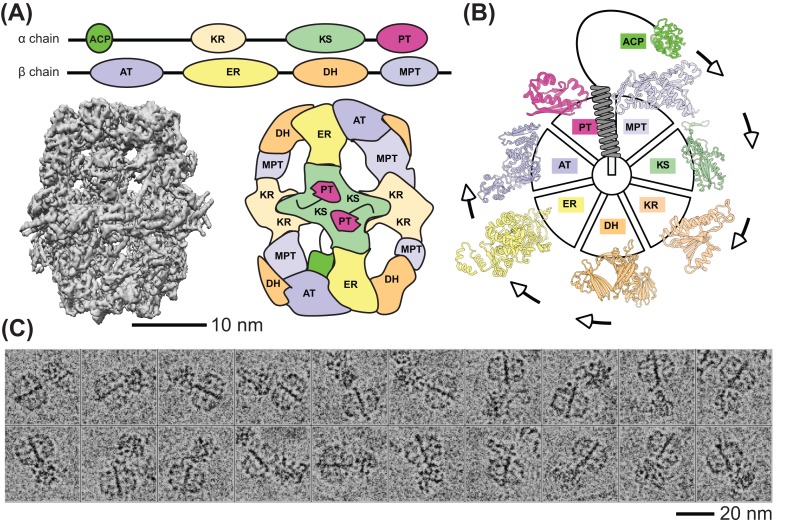
Figure 3. Substrate channeling mechanisms in FAS.
(A) Fungal FAS is composed of two chains, each of which includes four catalytic domains. These domains (explained in the Abbreviations list) are organized in a highly complex 2.6 MDa structure, and each is found six times in the structure, therefore forming 48-catalytic domain biomolecule, highly intricate in the organization of the catalytic domains. (B) A simplified mechanism of substrate shuttling in fungal FAS, where the acyl carrier protein (ACP) brings the newly synthesized fatty acid sequentially to each of the catalytic domains of FAS. Note that this wheel-like catalysis is present six times in the protein complex, and therefore, highly intricate allosteric communication is expected, which by now is completely unknown. (C) Single-particles from acquired cryo-electron micrographs ([2] and unpublished data) showing the direct interaction between FAS and various other protein complexes, possibly forming a metabolon in fatty acid metabolism. The biomolecules often show connecting densities, clearly illustrating the interconnectivity of large protein complex assemblies and their direct interaction.