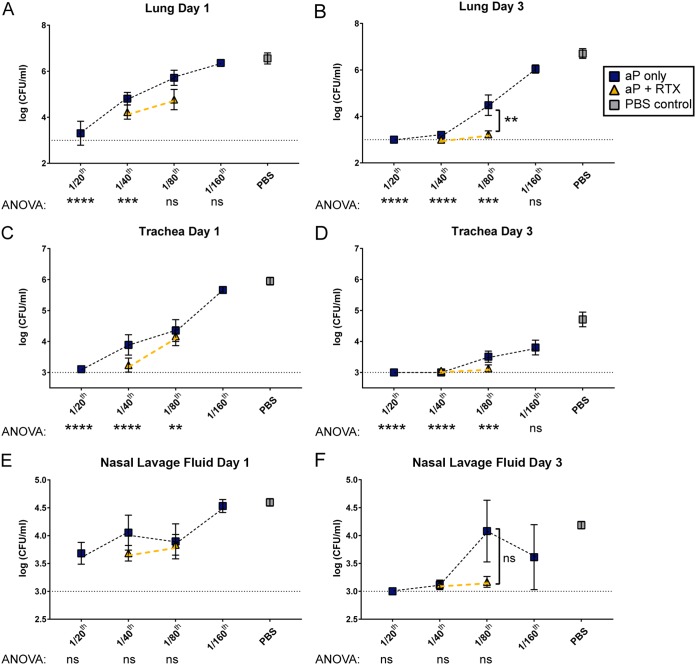
FIG 2.
Respiratory tract bacterial burden of CD-1 mice immunized with aPs or aPs supplemented with RTX antigen. Immunization with 1/20, 1/40, 1/80, and 1/160 human dose of aP was used to titrate the minimal effective dose and the maximum noneffective dose. RTX (5.6 μg) was included in 1/40 and 1/80 aP doses. Vaccinated CD-1 mice were challenged with 2 × 107 CFU of B. pertussis, and bacterial burden was determined at days 1 and 3. CFU counts were determined from lung homogenate (A and B), trachea homogenate (C and D), and nasal lavage fluid (E and F). Results are means ± SEM (n = 4 to 8, with 4 technical replicates) from independent experiments. ns, not significant; ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; *, P < 0.05. P values were determined by one-way ANOVA with Tukey's post hoc test and compared to naive mice (PBS control). The dashed line represents the lower limit of detection due to plating. The blue boxes indicate aP groups, whereas aP-plus-RTX groups are indicated by gold triangles. Gray squares indicate mock-vaccinated and -challenged mice. In panels E and F there were no statistically significant comparisons between the aP titration doses, as determined by ANOVA.