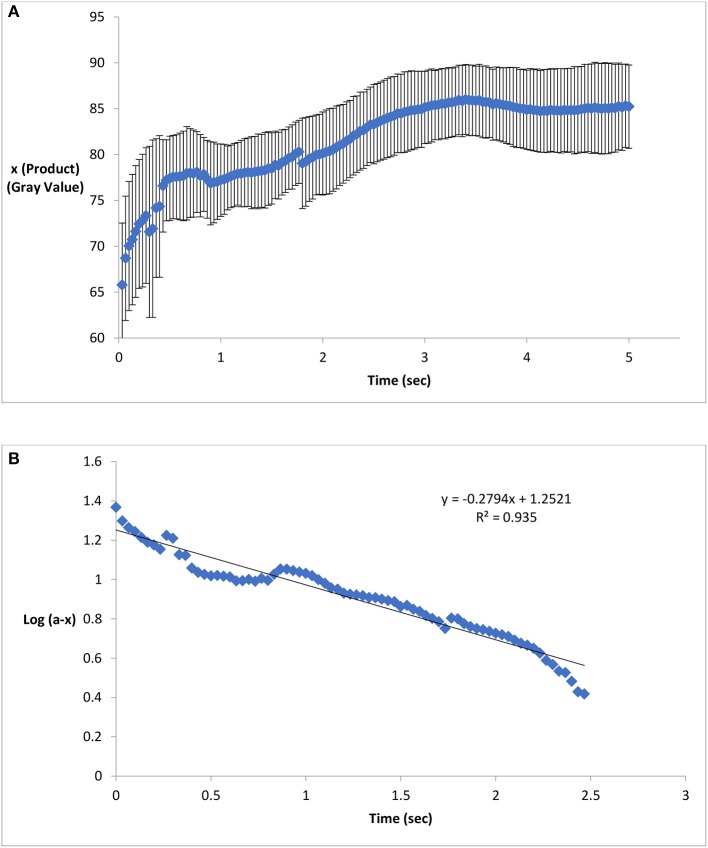
Figure 3.
Product formation and reaction kinetics on chemical treated paper surface. (A) Formation of Prussian blue on activated paper as a function of time. The color intensity of the product increased non-linearly as a function of time. (B) A straight line of Log (a-x) vs. time graph conforms first order reaction kinetics and the slope of this straight line indicates the reaction rate (Standard deviation bars for n = 5 samples).