Figure 6.
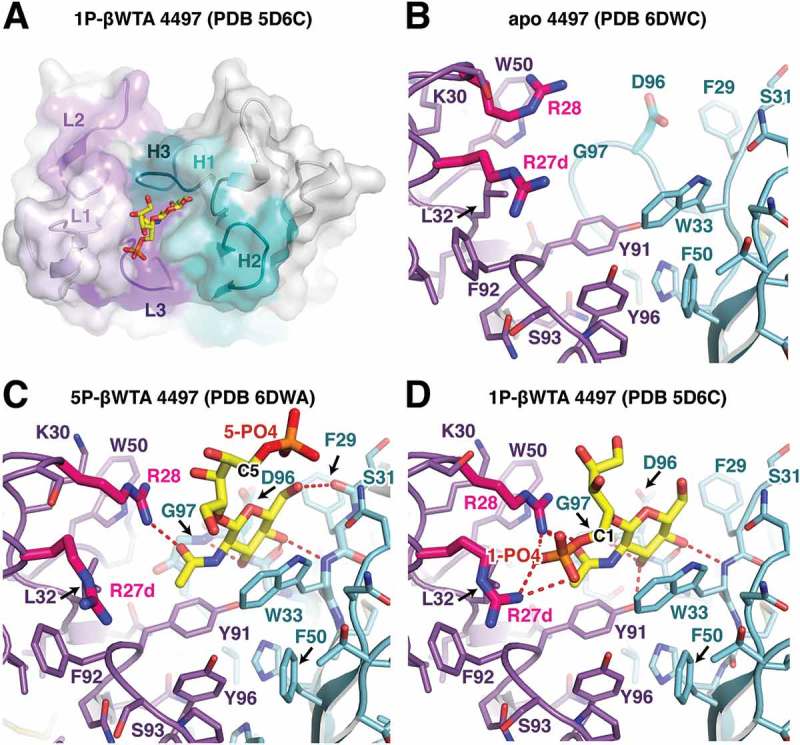
Structural analysis of the 4497 antibody. (A) Overview of the CDR interaction surface of the 4497 Fab in complex with 1-phosphate WTA (PDB ID 5D6C). The molecular surface is displayed as partially transparent to show CDR amino acids underneath. Light chain CDRs are labeled and colored in shades of purple, while heavy chain CDRs are labeled and shown in shades of cyan. The bound 1-phosphate β-WTA is shown in yellow as a stick representation. (B) Close-up view of the CDRs in the apo structure of 4497. CDR loops are shown as sticks and colored as in (A). (C, D) Close-up view of the interactions between the (C) 5-phosphate β-WTA or the (D) 1-phosphate β-WTA (PDB ID 5D6C) and the 4497 CDRs. CDR loops are shown as sticks and colored as in (A) with polar contacts shown as red dashed lines. CDR L1 residues Arg27d and 28, which contact the 1-phosphate, are shown in magenta to highlight changes in conformation between the apo- and WTA-bound structures.
