Figure 1.
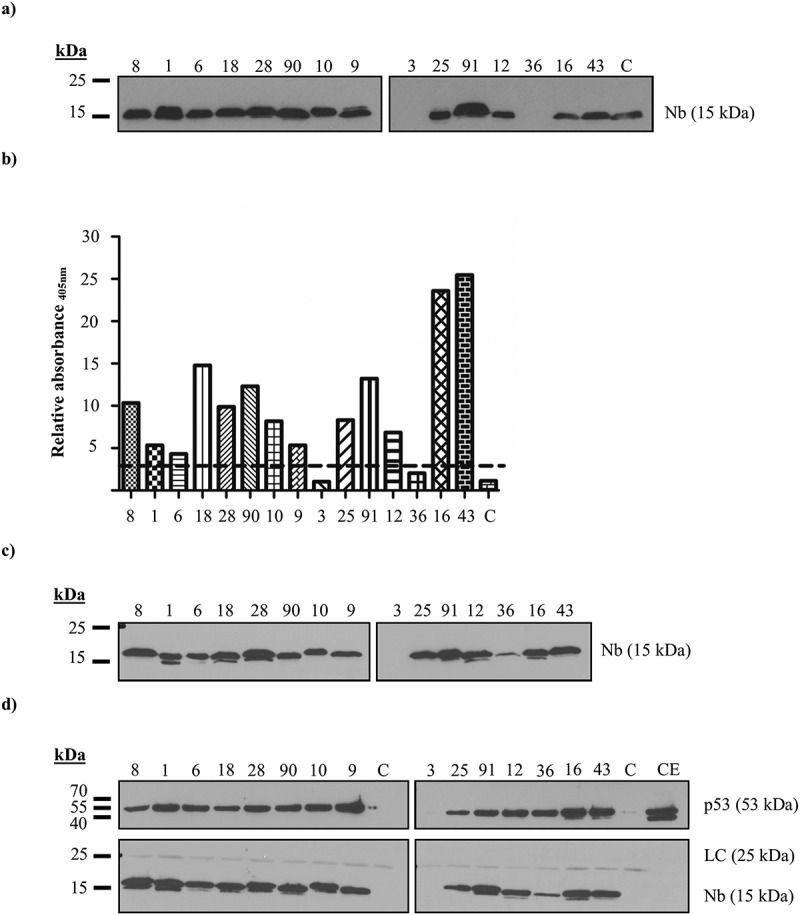
In vitro characterization of p53 TAD nanobodies. (a) Expression levels of the recombinant p53 TAD Nbs in the periplasmic extract. A total amount of 10 µg of nanobody-containing periplasmic extract was loaded onto a 15% SDS gel. An anti-HA antibody was used to visualize the Nbs. High expression yields are observed for 13 out of 15 Nbs, whilst the recombinant production for p53 TAD Nb3 and p53 TAD Nb36 is unsuccessful. (b) Results of ELISA experiment conducted with recombinant p53 TAD Nbs. A 96-well plate, coated with purified p53 TAD (1 µg/ml) or Annexin V (1 µg/ml), was incubated with 20 µl periplasmic extract, containing recombinant p53 TAD Nbs. An irrelevant nanobody was implemented as negative control (C). Nbs were considered as positive binders if the absorbance (at 405 nm) measured for the positive p53 TAD-coated wells exceeds those for the negative Annexin V-coated control wells by a factor of three (represented by a dashed line). All Nbs, with the exception of p53 TAD Nb3 and p53 TAD Nb36 whose recombinant expression were unsuccessful, meet the criterion. (c) Expression levels of the recombinant p53 TAD Nbs in the periplasmic extract. A total amount of 10 µg of nanobody-containing periplasmic extract was loaded onto a 15% SDS gel. An anti-HA antibody was used to visualize the Nbs. High expression yields are observed for 13 out of 15 Nbs, p53 TAD Nb36 displays lower yields, whilst recombinant production of p53 TAD Nb3 is again unsuccessful. (d) In vitro pull-down experiment performed with recombinant HA-tagged p53 TAD Nbs on endogenous p53 originating from crude extract of HEK293T cells. αHA- agarose beads were used for immobilization of the HA-tagged Nbs. A negative control was included where αHA- agarose beads were incubated with crude extract of HEK293T cells in the absence of a HA-tagged nanobody (C). A 40 µg sample of crude extract (CE) was also loaded onto the 15% SDS gel. An anti-HA antibody was used to visualize the Nbs and DO-1 was used to visualize p53. (LC = light chain of IgG antibody).
