Table 1.
Acetylcholinesterase inhibitory activity of 3-aminocoumarin derivatives.
| structure | compound | R | X | n | IC50 (nM) ± SDa,b |
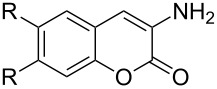 |
3 | H | – | – | 12%c |
| 8 | OMe | – | – | 5%c | |
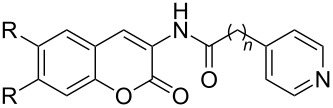 |
4 | H | – | 0 | 0%c |
| 5 | H | – | 1 | 8%c | |
| 9 | OMe | – | 0 | 9%c | |
| 10 | OMe | – | 1 | 9%c | |
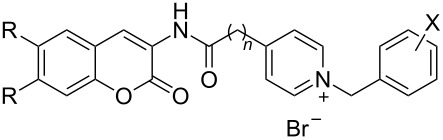 |
4a | H | H | 0 | 71.88 ± 3.44 |
| 5a | H | H | 1 | 10%c | |
| 9a | OMe | H | 0 | 12.48 ± 0.71 | |
| 10a | OMe | H | 1 | 1087.7 ± 0.05 | |
| 9b | OMe | 2-Cl | 0 | 6.03 ± 0.18 | |
| 9c | OMe | 3-Cl | 0 | 11.47 ± 0.63 | |
| 9d | OMe | 4-Cl | 0 | 293.17 ± 13.57 | |
| 9e | OMe | 2-F | 0 | 3.05 ± 0.28 | |
| 9f | OMe | 3-F | 0 | 5.04 ± 0.26 | |
| 9g | OMe | 4-F | 0 | 5.31 ± 0.38 | |
| 9h | OMe | 2,3-di-F | 0 | 1.53 ± 0.01 | |
| 9i | OMe | 2,6-di-F | 0 | 2.43 ± 0.18 | |
| donepezil·HCl | – | – | – | 53.51 ± 3.12 | |
| tacrine | – | – | – | 190.37 ± 4.55 | |
aConcentration of the compound that produced 50% inhibition of enzyme activity.
bResults are expressed as mean ± standard error with the average of triplicate independent experiments.
c% Inhibition at concentration of 1 μM.
