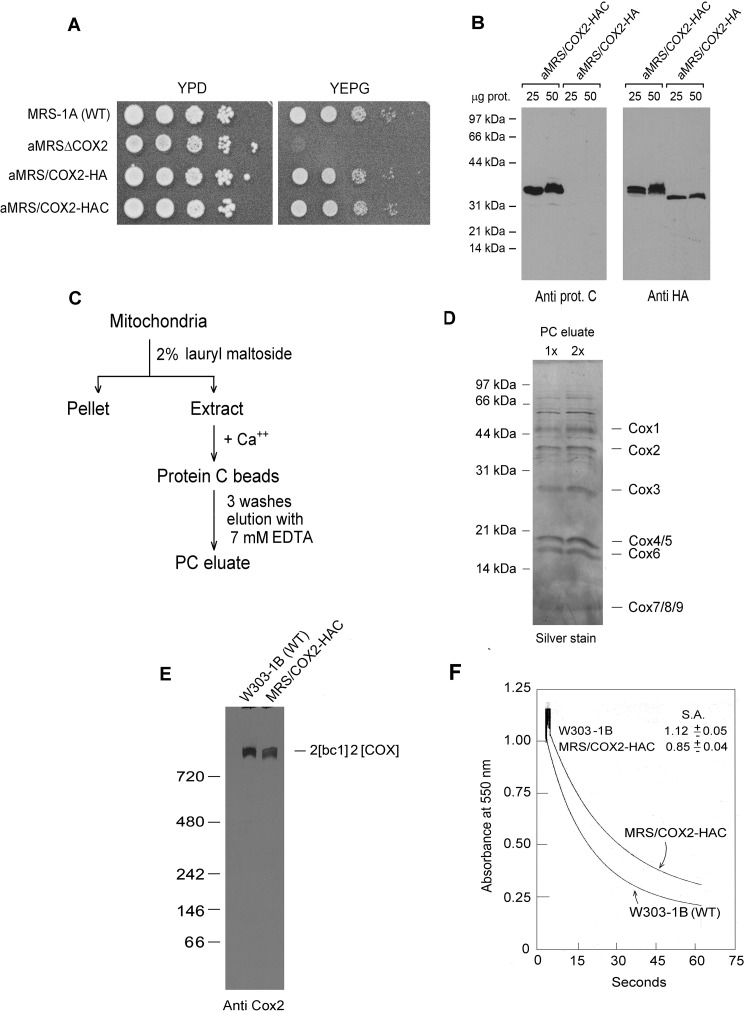
Figure 1.
Properties of yeast expressing HA- and HAC-tagged Cox2p. A, overnight cultures of the parental WT MRS-1A, aMRSΔCOX2, aMRS/COX2-HA, and aMRS/COX2-HAC were serially diluted and spotted on rich glucose (YPD) and rich glycerol/ethanol (YEPG) plates. The photograph was taken after 2 days' incubation at 30 °C. B, mitochondria were prepared from aMRS/COX2-HA and aMRS/COX2-HAC. The indicated amounts of mitochondria were applied to a 12% polyacrylamide gel and separated by SDS-PAGE. Western blots were incubated with polyclonal antibody against the protein C epitope followed by secondary peroxidase-coupled antibody against rabbit γ globulin. Proteins were visualized with SuperSignal chemiluminescent substrate kit (Pierce). C, protocol used to purify HAC-tagged cytochrome oxidase. D, two different concentrations of cytochrome oxidase purified as in C were depolymerized in Laemmli sample buffer (43) and separated by SDS-PAGE on a 12% polyacrylamide gel. Proteins were stained with silver. The subunits of cytochrome oxidase are identified in the right-hand margin. E, mitochondria from the indicated strains were extracted and separated by BN-PAGE. Proteins were transferred to a PVDF membrane, reacted with a polyclonal antibody against Cox2p and further processed as in B. F, mitochondria of the WT strain W303–1B and MRS/COX2-HAC expressing tagged Cox2p were assayed for oxidation of ferrocytochrome c at 550 nm. S. A. refers to specific activity expressed as micromoles of cytochrome c oxidized per minute per milligram protein.