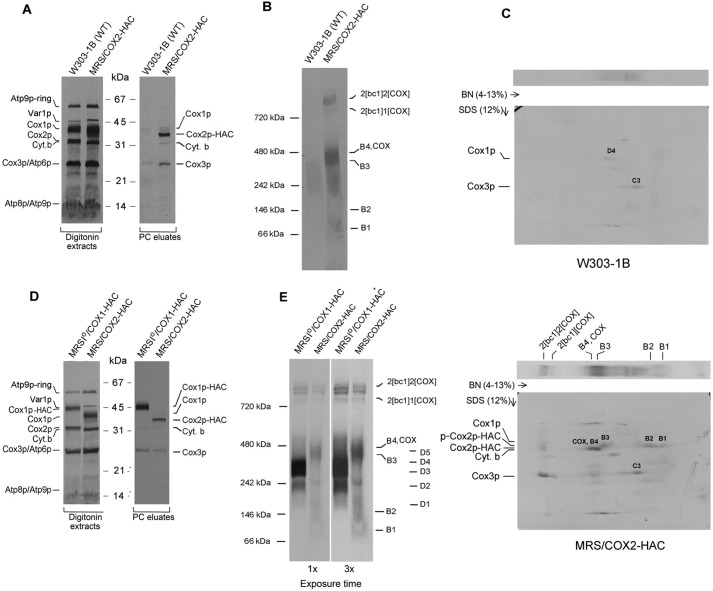
Figure 2.
In organello labeling of Cox2p intermediates. A, mitochondria were isolated from respiratory competent haploid strains W303–1B and MRS/COX2-HAC. Mitochondria (250 μg protein) were labeled in vitro with [35S]methionine plus [35S]cysteine and extracted with digitonin as described previously (10). The digitonin extract (5% of total) and the fraction purified on protein C antibody beads as in Fig. 1D (15% of total) were separated by SDS-PAGE on a 12% polyacrylamide gel, transferred to nitrocellulose and exposed to X-ray film. B, the fraction purified on the protein C beads was separated by BN-PAGE on a 4–13% polyacrylamide gel, transferred to a PVDF membrane, and exposed to X-ray. C, the purified PC eluates from WT and from MRS/COX2-HAC were separated by BN-PAGE on a 4–13% polyacrylamide gel in the first dimension and by SDS-PAGE in the second dimension. The background bands detected in the autoradiogram of the WT control (2D gel of top panel) correspond to the D3 and D4 intermediates of the Cox1p module and the C3 intermediate of Cox3p module. The radiolabeled bands pulled down by Cox2p are the B1, B2, and B3 intermediates. The Cox2p precursor (p-Cox2p), the B4 intermediate and COX with mature Cox2p and the nonspecific adsorbed C3 intermediate with Cox3p (2D gel of lower panel). The migration of Cox1p, Cox2p-HAC, and the Cox2p precursor are marked in the margins. D and E, same as A and B, except that MRSIO/COX1-HAC, a strain expressing Cox1p with an HAC tag was used for comparison. The Western blotting of the native gel in the right panel was exposed three times longer.