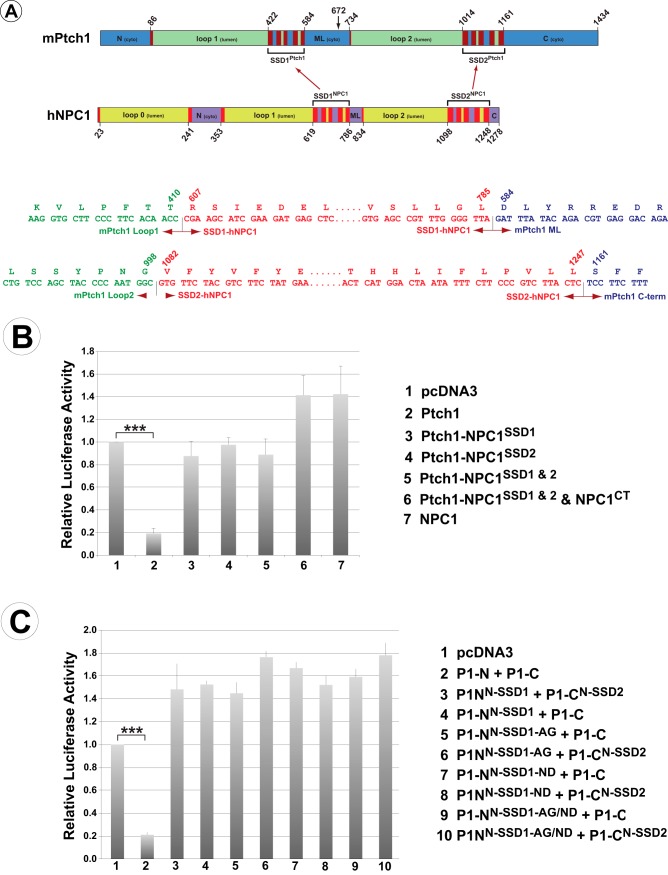
Figure 7.
The SSDs from NPC1 cannot restore Ptch1 activity. A, stick diagram illustrating the specific amino acid boundaries of the Ptch1 and NPC1 constructs used to create chimeric Ptch1 proteins with the SSDs from NPC1. The arrow at aa 672 indicates the break point separating the two half-molecules used in C. DNA and amino acid sequences for the specific boundaries of the chimeric proteins are also shown. B, Ptch1 with individual or both SSD regions replaced with the analogous regions of NPC1 were tested in the Hh repression assay. None of the constructs tested were able to repress Smo-dependent Hh signaling, thus demonstrating that the SSDs from these distinct classes of SSD-containing proteins do not act as generic modules. C, two amino acids necessary for Ptch1 function, which are different in NPC1 (Gly495 and Asp500 in mPtch1), were mutated to those in the identical position in Ptch1. This was done in a chimeric protein encoding the N-terminal half of Ptch1 but with SSD1 replaced with that of NPC1 (PNS1-N). These were co-expressed with the WT C-terminal half of Ptch1 (Ptch1-C) or the C terminus with the second SSD replaced with that from NPC1 (PNS2-C) and tested for their ability to repress Smo-dependent Hh activity. Changing these specific amino acids was not sufficient to restore Smo repression activity. ***, p < 0.001.