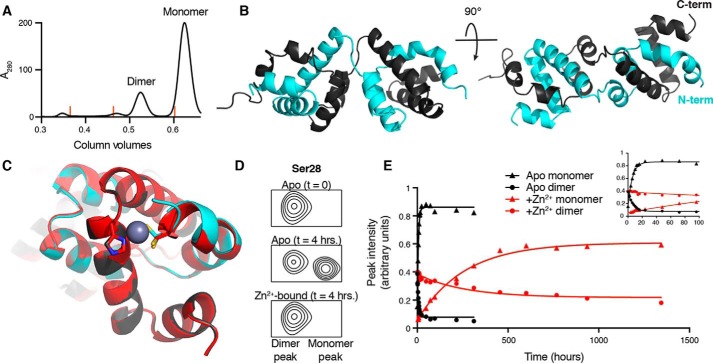
Figure 5.
The CARD9–CARD can adopt a 3-helix domain-swapped dimer in solution. A, size-exclusion chromatography UV trace (280 nm), demonstrating the presence of both monomeric and dimeric CARD9–CARD when purified from E. coli. Orange lines indicate peak positions of molecular mass standards of 158, 44, and 17 kDa. B, crystal structure of the domain-swapped dimer, with the two polypeptide chains colored in gray or cyan. C, alignment of the apo (gray/cyan) and Zn2+-bound (red) domain-swapped dimer structures with Cys-10 and His-73 depicted as sticks. Cys-10 in the apo-form adopts two distinct rotameric states in the crystal structure, both of which are depicted. D, 15N-SOFAST-HMQC spectra, beginning with 100% CARD9–CARD dimer, focused on the representative backbone amide peak of Ser-28, immediately (top) and 4 h (middle, without Zn2+, bottom, with Zn2+) after beginning incubation at 25 °C. See Fig. S3C for full spectra. E, Ser-28 monomer (triangles) and dimer (circles) peak heights as a function of time in the apo (black) or Zn2+-bound (red) states. Inset plot includes only the first 100 h of the experiment, allowing visualization of the exponential approach to equilibrium in the apo state.