Figure 1.
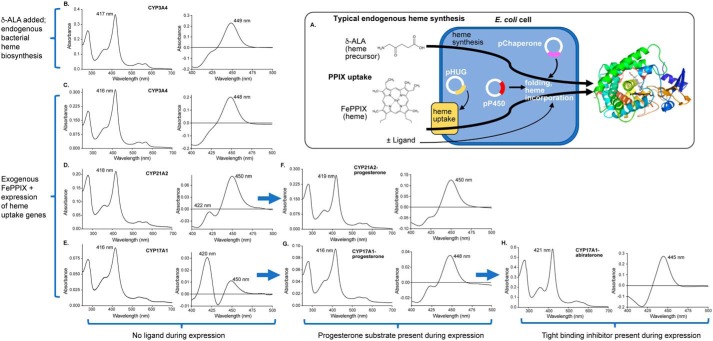
Generation of three human membrane cytochrome P450 enzymes employing exogenous FePPIX. A, typical membrane P450 recombinant expression involves E. coli growth in rich medium supplemented by the heme precursor δ-aminolevulinic acid (δ-ALA), which is cell-permeable and supports endogenous bacterial heme synthesis for incorporation into the P450 polypeptide. The new method supports the possibility of P450 nonnative metal incorporation by growing E. coli in iron-depleted minimal medium supplemented with PPIX containing iron or other metals. Expression of four heme uptake genes from the pHUG plasmid facilitates uptake of this PPIX for incorporation into the P450 polypeptide. Both methods employ coexpression of chaperone proteins to facilitate folding and increase P450 yield. The usual endogenous heme biosynthesis method results in CYP3A4 protein (B) with a Soret peak consistent with water coordination to the heme iron (417 nm), whereas the reduced-carbon monoxide difference spectrum indicates mostly P450 and little P420. Very similar results are observed for CYP3A4 expressed with exogenous supplementation of FePPIX (C). However, the same exogenous FePPIX method results in purified CYP21A2 (D) and CYP17A1 (E) with normal absolute spectra (416–418 nm), but significant amounts of undesirable P420. The addition of the substrate progesterone in the medium during expression with exogenous FePPIX results in CYP21A2 (F) and CYP17A1 (G) in which the substrate has dissociated during purification (Soret at 416–419 nm) but which has increased yield (Table 1) and reduced amounts of P420. The addition of the tight-binding inhibitor abiraterone during expression of CYP17A1 with exogenous FePPIX (H) yields protein in which the abiraterone pyridine is still bound to the heme iron after purification (Soret at 421 nm), further increases yield (Table 1), and prevents P420 formation.