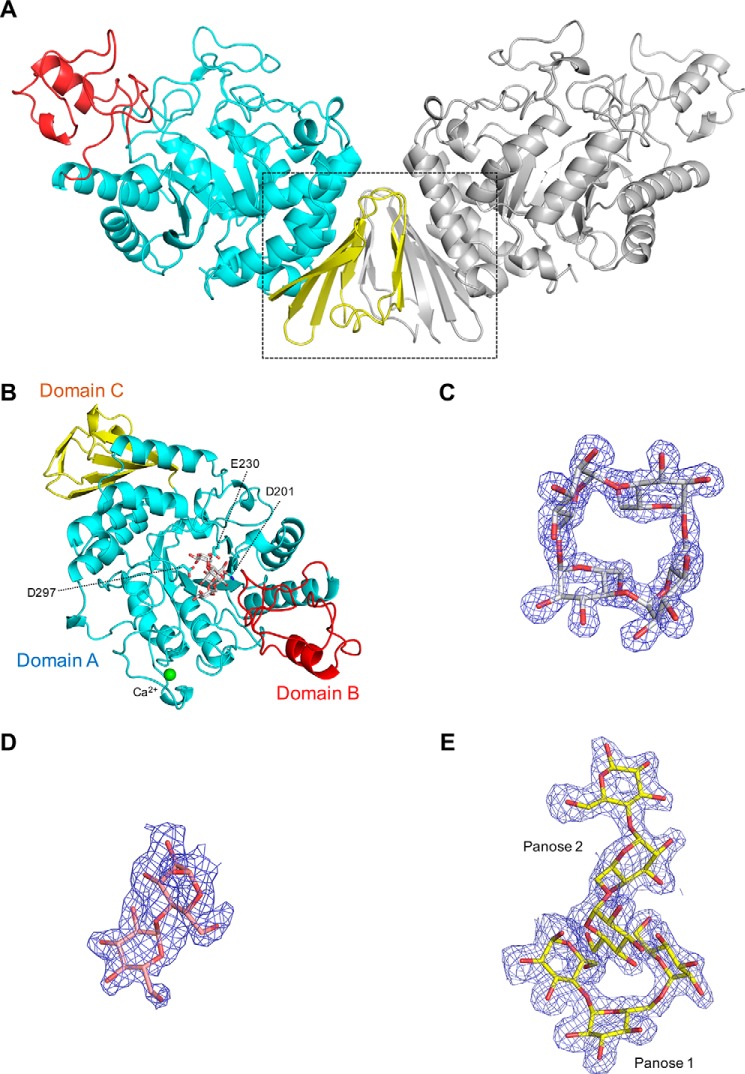
Figure 2.
Overall structure of CMMase and electron density maps of ligands. A, dimer structure in the crystals. One monomer is colored by domain (catalytic domain A in cyan, domain B in red, and domain C in yellow), and the other monomer is shown in gray. B, monomer structure of CMMase–CMM complex. The catalytically important residues (Asp-201 as nucleophile, Glu-230 as acid/base, and Asp-297 as fixer), bound CMM, and a calcium ion are shown as cyan sticks, gray sticks, and a green sphere, respectively. C–E, mFo − Fc omit electron density maps of CMM (C, contoured at 4.0 σ), maltose (D, 2.0 σ), and panose (E, 2.0 σ), in the complex structures.