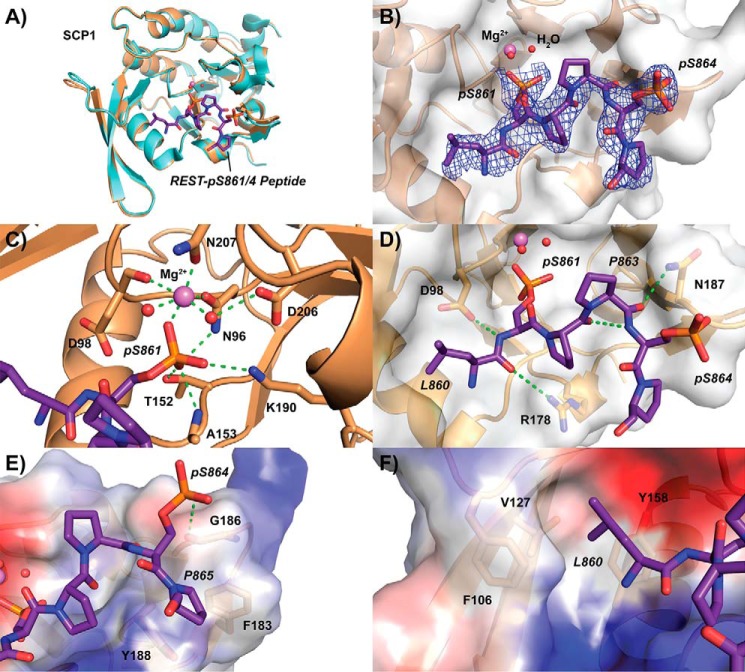
Figure 2.
Crystal structure of SCP1 bound to REST pSer-861/864 peptide. A, overall structure of SCP1 (sand) when bound to REST peptide (purple) compared with the apo structure (light blue). Magnesium (magenta) and active site waters (red) are represented as spheres. B, electron density of refined pSer-861/864 bound peptide. The 2mFo − DFc map representing electron density is contoured to 1σ and illustrated as a blue mesh around the REST peptide. The Van der Waals exterior of SCP1 is represented as a transparent gray surface. C, phosphoserine-binding site of SCP1 occupied by pSer-861 of the doubly phosphorylated REST peptide. Hydrogen bonding in the magnesium coordination pocket is represented as dotted green lines. D, tight hairpin configuration of the pSer-861/864 peptide stabilized by internal and external hydrogen bonding. E, binding of the C-terminal residues of the doubly phosphorylated REST peptide to the periphery of the SCP1 pocket. Surface electrostatic potentials are denoted by color (blue, positive; red, negative; gray, neutral). F, hydrophobic pocket binding to the side chain of Leu-860 of the REST peptide.