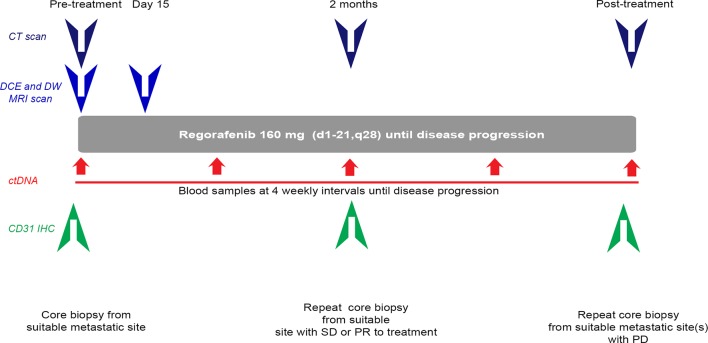
Figure 1.
PROSPECT-R trial design. Patients meeting all inclusion and no exclusion criteria were required to have pretreatment CT, DCE-MRI and DW-MRI scans; MRI scans were then repeated on day 15. All patients were also required to have pretreatment mandatory core biopsy, followed by a core biopsy at 2 months if they had SD or PR. Patients were monitored by CT scans every 2 months until the time of PD and if clinically feasible, they had biopsy of one or two progressing lesions from PD sites. Plasma samples were collected every 4 weeks until the time of PD. ctDNA, circulating tumour DNA; DCE, dynamic contrast enhanced; DW, diffusion weighted; MRI, magnetic resonance imaging; PD, progressive disease; PR, partial response; SD, stable disease.