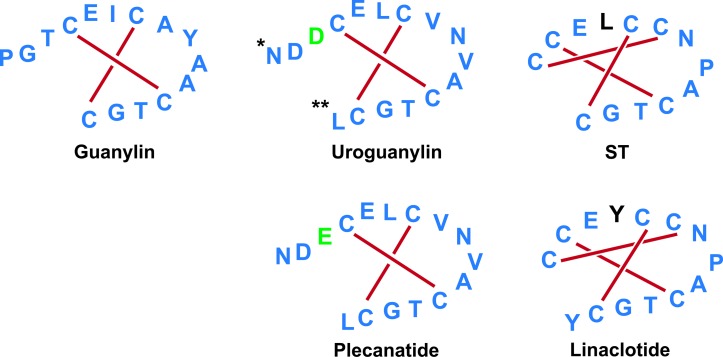
Figure 3.
Structures of GC-C agonists. Both uroguanylin and guanylin are active endogenous ligands of GC-C. These peptides have many structural conformations defined by two disulfide bonds, in striking contrast to the three disulfide bonds and rigid structure of STs. Further, uroguanylin has pH-sensing aspartate residues in its N terminus (*), which allow the ligand to preferentially bind GC-C in acidic (pH 5–6) environments of the proximal small intestine, as well as a COOH-terminal leucine (**) compared with guanylin. Plecanatide is the synthetic analogue of uroguanylin, retaining two intrachain disulfide bridges and two acid-sensing amino acids in the N terminus, which together confer preferential biological activity in acid pH environments. In contrast, linaclotide is the synthetic analogue of STs, retaining three intrachain disulfide bridges in the absence of acid-sensing residues in the N terminus, conferring a rigid structure whose activity is pH independent.