Figure 2.
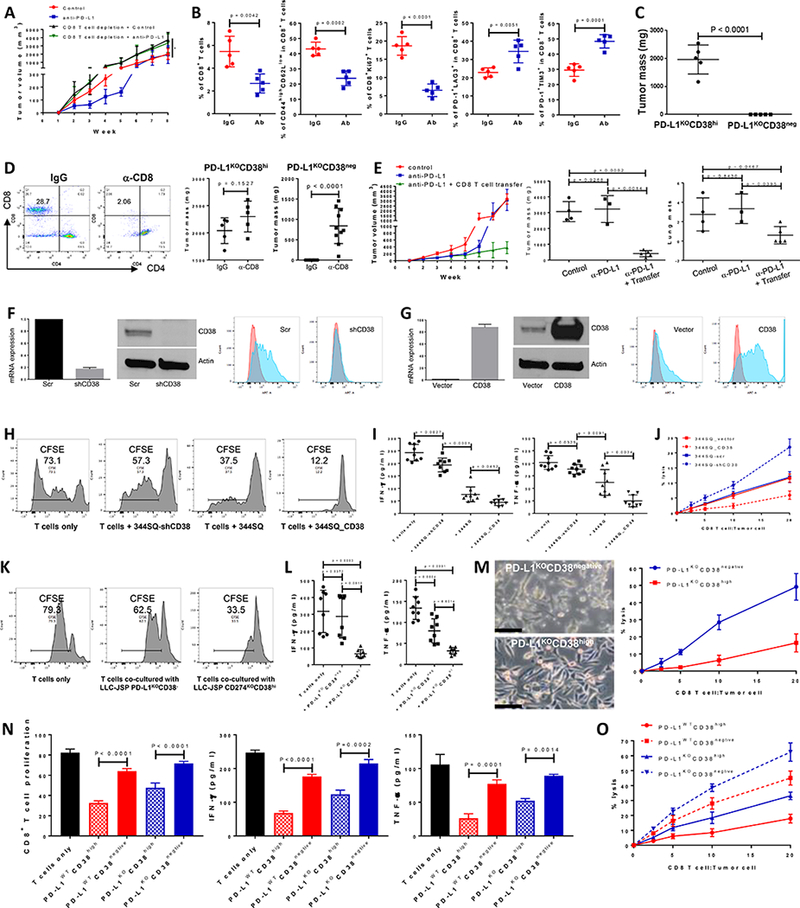
CD38 on tumor cells suppresses CD8+ T cell function.
(A) Growth of subcutaneous 344SQ tumors in immune competent 129/Sv mice treated with IgG control, anti-PD-L1 (clone 9G2; 200 μg per mouse), anti-PD-L1 plus anti-CD8 (clone 2.43; 200 μg per mouse), respectively. Mice (n = 5 or 8) were intraperitoneally treated with the antibody once a week for 7 weeks beginning on day 7 after the tumor cell injection (1 × 106 cells per mouse). In the group of anti-PD-L1 plus anti-CD8 treatment, mice were pretreated with anti-CD8 antibody (400 μg per mouse) one week before tumor cell injection. Tumor sizes are presented as mean ± SEM and statistical significance (ns, no significant difference; *p < 0.05; **p < 0.01) determined on indicated weeks. The statistically significant differences between the groups of control and anti-PD-L1 are as follows: week 1, ns; week 2, *; week 3, **; week 4, *; week 5, **; week 6, *; week 7, ns; week 8, ns. ANOVA test was used to calculate the significant difference between two groups. For the analysis among multiple groups at the end point (week 8), ANOVA was used to analyze, *p < 0.05.
(B) FACS analysis of % CD8+TIL, the proliferation marker Ki67, surface CD44, CD62L, PD1, LAG3, and TIM3 marker expression levels on CD8+ T cells from primary tumors in 129/Sv mice (n = 5) treated with anti-PD-L1 antibody at week 5. Data are shown as mean ± sd. t-test was used to analyze.
(C) PD-L1KOCD38high531LN3 cells or PD-L1KOCD38negative531LN3 cells (5 × 106 cells per mouse) were subcutaneously injected into immune competent 129/Sv mice (n = 5). The primary tumor sizes at week 4 are shown with mean ± SEM. t-test was used to analyze.
(D) (Left panel) CD8 T cells in spleen were determined 2 weeks post initial anti-CD8 antibody injection to test CD8 T cell depletion efficiency. (Middle panel) 5 × 106 of PD-L1KOCD38high531LN3 cells were subcutaneously injected into 129/Sv mice (n = 5) after CD8 T cells were depleted (α-CD8). The T cell un-depleted group was included as the control (IgG). The total tumors were measured 4 weeks post tumor cell transplantation and are shown with mean ± SEM. t-test was used to analyze. (Right panel) 5 × 106 of PD-L1KOCD38negative 531LN3 cells were subcutaneously injected into 129/Sv mice (n = 10) after CD8 T cells were depleted (α-CD8). The T cell un-depleted group was included as the control (IgG). The total tumors from primary site and peritoneal cavity were measured 5 weeks post tumor cell transplantation and are shown with mean ± SEM. t-test was used to analyze.
(E) To prepare CD8+ T cells, 129/Sv mice were challenged with 0.5 × 106 344SQ for 2 weeks. CD8+ T cells were isolated from these tumors, blood, and spleens. A separate cohort of 344SQ tumor-bearing mice were treated with anti-PD-L1 antibody or control (as described in Figure 1A), then used as recipients for the CD8 T cell adoptive transfer assay. At week 4, mice received cyclophosphamide at 100 mg/kg intravenously 6 hours before CD8 T cell transfer (6 × 106 per mouse, intravenously), followed by IL-2 (20,000 units, intraperitoneally) at 8 hours after T cell transfer then every 12 hours for 3 days. The tumor growth curves are shown in the left panel. At the endpoint, mice were necropsied to harvest primary tumors and lungs, which were weighed, and to quantify distant metastases. The primary tumor weights and lung metastatic nodules are shown in the middle and right panels. ANOVA test was used to analyze.
(F) Cell lines with stable expression of a scramble control (344SQ-scr) or shRNA against CD38 (344SQ-shCD38) were generated and subjected to qPCR assays. Relative CD38 mRNA levels are normalized to L32 and shown in the left panel, the Western blot assay for protein in the middle panel, versus β-actin as a loading control. The surface expression of CD38 on the cell lines was determined using FACS and are shown in the right panel.
(G) The stable cell lines 344SQ_vector (empty vector control) and 344SQ_CD38 (CD38 overexpression) were generated and subjected to qPCR assays. mRNA levels of CD38 are normalized to L32 and shown in the left panel, Western blot assay for protein in the middle panel, versus β-actin as a loading control. The surface expression of CD38 was determined using FACS and is in the right panel.
(H-J) To prepare tumor specific CD8+ T cells, 129/Sv mice were challenged with 0.5 × 106 344SQ for 2 weeks. CD8+ T cells were isolated from these tumors, blood, and spleens. CFSE-labeled CD8+ T cells were co-cultured with indicated cancer cells in the presence of anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) for 4 days. CD8+ T cells only was included as the control. T cell proliferation was quantified using FACS analysis (H). The supernatants from each co-culture were subjected to ELISA assay to measure IFN-γ and TNF-α (I). Tumor cells and CD8+ T cells were co-cultured at the indicated ratios in the presence of anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) for 4 days. Tumor cells only were used as the control for calculation. At day 4, CD8+ T cells and dead tumor cells were washed away and viable tumor cells were counted with 0.4% Trypan Blue staining. The CD8+ T cell killing efficiency is shown in (J). The experiments were repeated at least three times. p values were calculated with ANOVA test.
(K-M) To prepare tumor specific CD8+ T cells, C57BL/6 mice were challenged with 0.2 × 106 LLC-JSP for 2 weeks. CD8+ T cells were isolated from these tumors, blood, and spleens. CFSE-labeled CD8+ T cells were co-cultured with indicated cancer cells in the presence of anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) for 4 days. CD8+ T cells only was included as the control. T cell proliferation was quantified using FACS analysis (K). The supernatants from each co-culture were subjected to ELISA assay to measure IFN-γ and TNF-α (L). Tumor cells and CD8+ T cells were co-cultured at the indicated ratios in the presence of anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) for 4 days. Tumor cells only were used as the control for calculation. At day 4, after taking photos, CD8+ T cells and some dead tumor cells were washed away and viable tumor cells were counted with 0.4% Trypan Blue staining. The CD8+ T cell killing efficiency is shown in (M). The experiments were repeated at least three times. p values were calculated with ANOVA test. Scale bars represent 100 μm.
(N and O) To prepare tumor specific CD8+ T cells, 129/Sv mice were challenged with 0.5 × 106 531LN3 for 2 weeks. CD8+ T cells were isolated from these tumors, blood, and spleens. CFSE-labeled CD8+ T cells were co-cultured with indicated cancer cells (sorted from PD-L1WT531LN3 or PD-L1KO531LN3) in the presence of anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) for 4 days. CD8+ T cells only was included as the control. T cell proliferation was quantified using FACS analysis (N). The supernatants from each co-culture were subjected to ELISA assay to measure IFN-γ and TNF-α (N). Tumor cells and CD8+ T cells were co-cultured at the indicated ratios in the presence of anti-CD3 (5 μg/ml) and anti-CD28 (5 μg/ml) for 4 days. Tumor cells only were used as the control for calculation. At day 4, CD8+ T cells and some dead tumor cells were washed away and viable tumor cells were counted with 0.4% Trypan Blue staining. The CD8+ T cell killing efficiency is shown in (O). The experiments were repeated at least three times. p values were calculated with ANOVA test.
