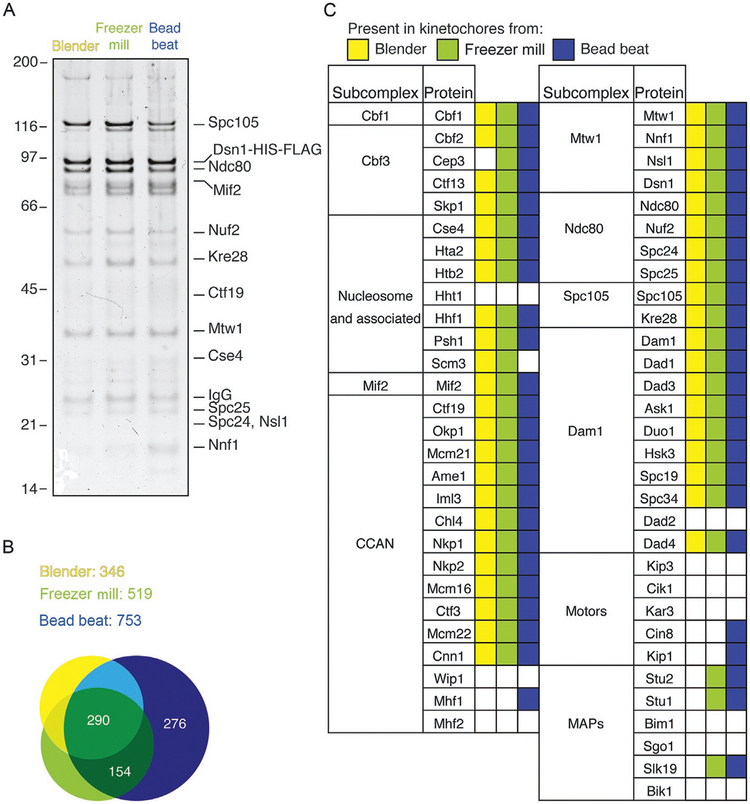
FIG. 3.
Lysis method affects the number of unique proteins coimmunoprecipitated with Dsn1. (A) Kinetochores purified from yeast extract by immunoprecipitation of Dsn1-HIS-FLAG for 3h at 4°C are visualized by silver-stained SDS-PAGE. (B) Shotgun mass spectrometric analysis was performed on kinetochore purifications from the three lysis methods of purification and the number of unique proteins detected is indicated. Purification from blender lysates yields the fewest unique proteins, followed by freezer mill, with bead beat yielding the highest number of different proteins coimmunoprecipitated. There is a large overlap of the three sets indicating that all three lysis methods yield kinetochores with differences in the additional components purified. (C) Summary of kinetochore composition comparing the three lysis methods shows that the vast majority of the known kinetochore components are detected by all methods. However, the bead beat lysis, and to a smaller extent the freezer mill lysis, yields more of the transiently associated outer kinetochore components.