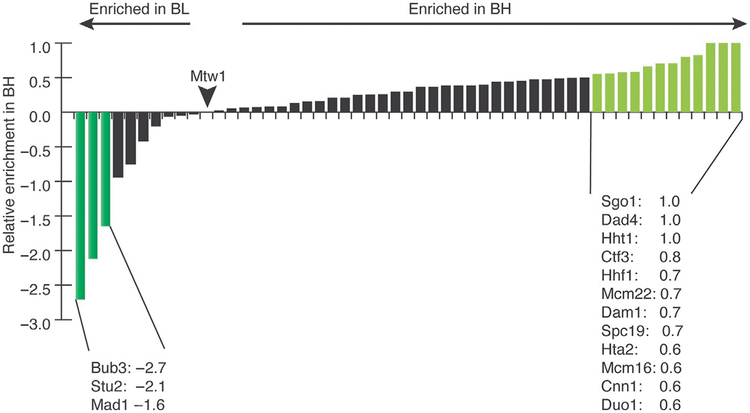
FIG. 4.
Purification in buffer BL 0.175 leads to enrichment of some transient components. Nonquantitative shotgun mass spectrometry was performed on kinetochores purified using the freezer mill lysis method in either buffer BH 0.15 or BL 0.175. The resulting number of total peptides for every protein was normalized to the number of peptides of the Mis12 complex component, Mtw1. To compare across the two sets, we plotted a relative enrichment of a certain protein in BH 0.15 compared to BL 0.175 using the formula: (normalized BH 0.15 peptides−normalized BL 0.175 peptides)/normalized BH 0.15 peptides. By this analysis, most proteins were slightly more enriched in BH 0.15 vs BL 0.175. There were 12 proteins that were more than twofold enriched in BH 0.15 and 3 proteins that were more than twofold enriched in BL 0.175. The proteins enriched in BH 0.15 can be classified into three broad categories: the Dam1 complex, the Ctf3-Cnn1 arm of the kinetochore, and histones/chromatin. The major proteins enriched with the buffer BL 0.175 are the SAC proteins and the MAP, Stu2.