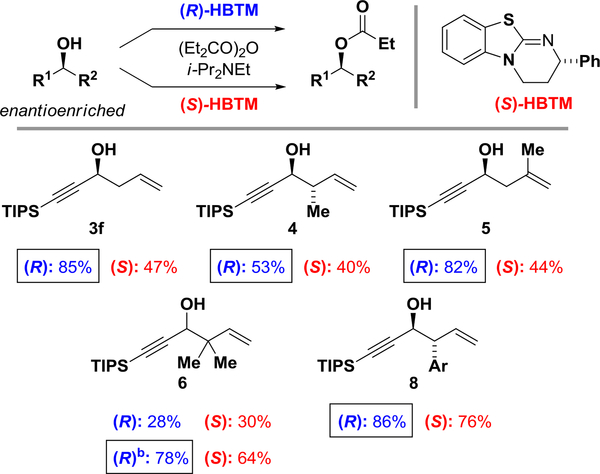
Scheme 2.
Analysis of alcohol configurations using the competing enantioselective conversion (CEC) method.a
aParallel acylation reaction were run with (R)- or (S)-HBTM catalyst (10 mol %), DIPEA (300 mol %) and propionic anhydride (300 mol %) at room temperature. The conversions were analyzed by 1H NMR after 1560 min. With the directing group to the left, faster reactions with the (R)HBTM indicate that the alcohol configuration is forward. See Supporting Information for more details. b0 °C, HBTM (26 mol %).