Table 1.
Enantioselective iridium-catalyzed allylation of ynals 1a-1f with allyl acetate via 2-propanol-mediated reductive coupling.a
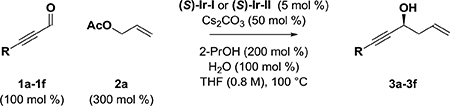 | ||||
|---|---|---|---|---|
| entry | 1a-1d | catalyst | yield (%) | ee (%) |
| 1 | 1a, R = Ph | (S)-lr-l | — | — |
| 2 | 1a, R = Ph | (S)-lr-ll | — | — |
| 3 | 1b, R = CH2OTBDPS | (S)-lr-l | — | — |
| 4 | 1b, R = CH2OTBDPS | (S)-lr-ll | — | — |
| 5 | 1c, R = CMe2OTIPS | (S)-lr-l | 63 | 95 |
| 6 | 1c, R = CMe2OTIPS | (S)-lr-ll | 53 | 94 |
| 7 | 1d, R = TMS | (S)-lr-l | — | — |
| 8 | 1d, R = TMS | (S)-lr-ll | — | — |
| 9 | 1e, R = TBS | (S)-lr-l | 24 | 94 |
| 10 | 1e, R = TBS | (S)-lr-ll | 22 | 96 |
| 11 | 1f, R = TIPS | (S)-lr-l | 78 | 95 |
| 12 | 1f, R = TIPS | (S)-lr-ll | 61 | 93 |
 | ||||
Yields are of material isolated by silica gel chromatography. Enantioselectivity was determined by chiral stationary phase HPLC analysis. See Supporting Information for further experimental details.
