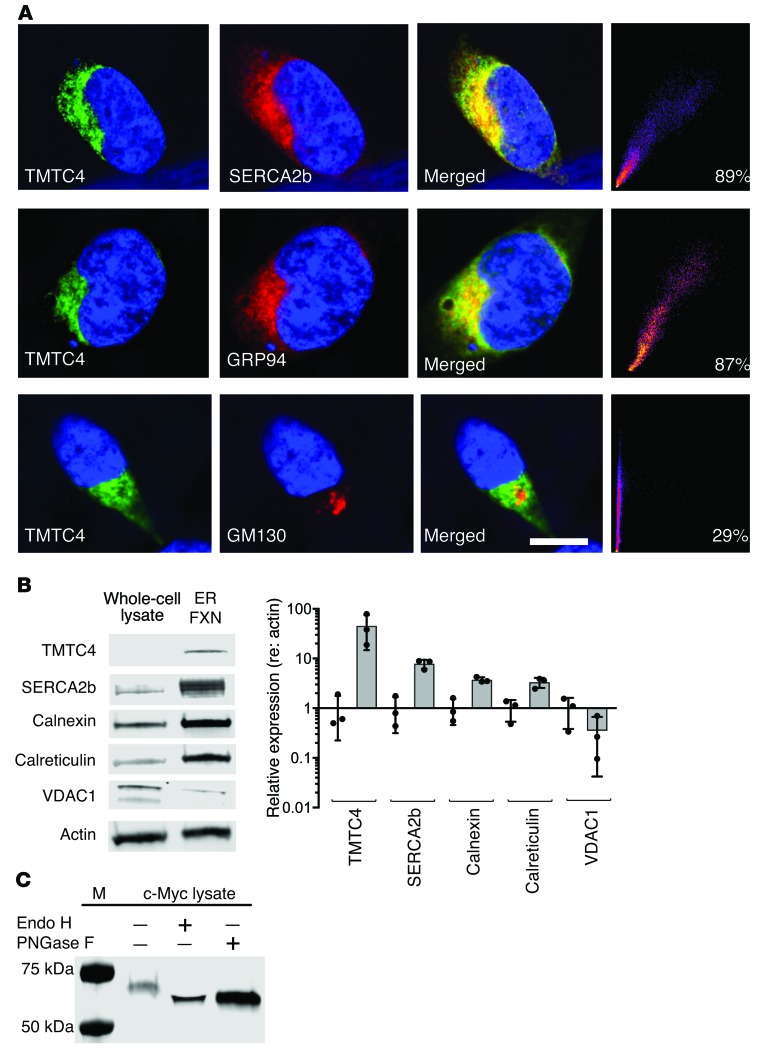
Figure 4. TMTC4 enrichment in the ER and colocalization with ER markers.
(A) HEK cells were stably transfected with TMTC4-c-myc constructs and stained with antibodies against TMTC4 (green, first column), ER proteins (GRP94 or SERCA2b), or the Golgi-enriched protein GM130 (red, second column), and DAPI to stain nuclei (blue, all panels). As demonstrated in the merged panels (third column), TMTC4 colocalizes with ER proteins but not with the Golgi protein (quantified in the fourth column). Images are representative of 5 experiments. Scale bar: 10 μm. (B) Nonnuclear whole-cell lysate (whole-cell lysate) from WT HEK cells, and a fraction enriched for ER membranes (ER Fxn), were stained with antibodies against TMTC4, as well as to the known ER resident proteins SERCA2b, calnexin, and calreticulin; all showed enrichment in the ER. As controls, actin was not enriched, and the mitochondrial membrane protein VDAC1 was barely detected, in the ER fraction. Quantification of expression levels (relative to actin, and normalized against the relative expression level in whole-cell lysate) confirm the ER localization of endogenous TMTC4 (n = 3 experiments, from which representative images were taken). (C) TMTC4-c-Myc and copurifying proteins were isolated from a postnuclear supernatant of HEK cells with stably incorporated TMTC4-c-Myc vector. This lysate (c-Myc lysate) was then treated with or without Endo H and/or PNGase F and the fractions assessed with antibody against c-Myc. The shift in molecular weight in TMTC4 treated with both Endo H and PNGase F indicates that TMTC4 is glycosylated, consistent with residence in the ER. M: molecular weight markers. Images are representative of 3 experiments.