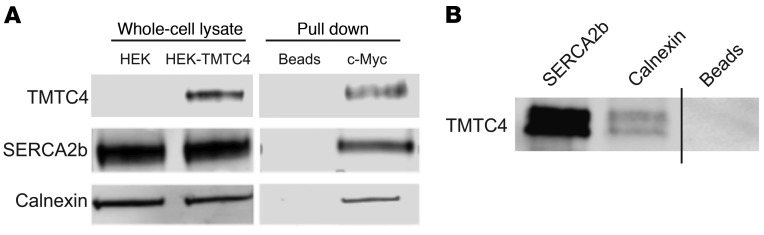
Figure 5. TMTC4 coimmunoprecipitation with ER membrane proteins.
(A) Nonnuclear whole-cell lysates from HEK cells with stably incorporated TMTC4-c-Myc vector (HEK-TMTC4) or empty plasmid (HEK) were assayed with antibodies against ER proteins (SERCA2b, calnexin) or TMTC4, showing that all 3 proteins were present in transfected HEK cells and that the TMTC4 antibody did not stain nonspecifically in mock-transfected cells. These extracts from TMTC4-c-Myc stably transfected cells were then incubated with agarose beads linked with antibodies against c-Myc or to beads alone (Beads). Bound proteins were purified and the “pull down” fraction stained with the aforementioned antibodies against TMTC4 and ER proteins, showing that SERCA2b and calnexin are copurified upon c-Myc pull down. Images are representative of 3 experiments. (B) Human fetal brain lysate was used to assess endogenous TMTC4 interactions. Pull down fractions were isolated using beads linked to antibody against SERCA2b, calnexin, or beads alone without linked antibodies (Beads), and probed with antibody against TMTC4. Endogenous TMTC4 was found to coimmunoprecipitate strongly with SERCA2b and weakly with calnexin. Images are representative of 3 experiments.