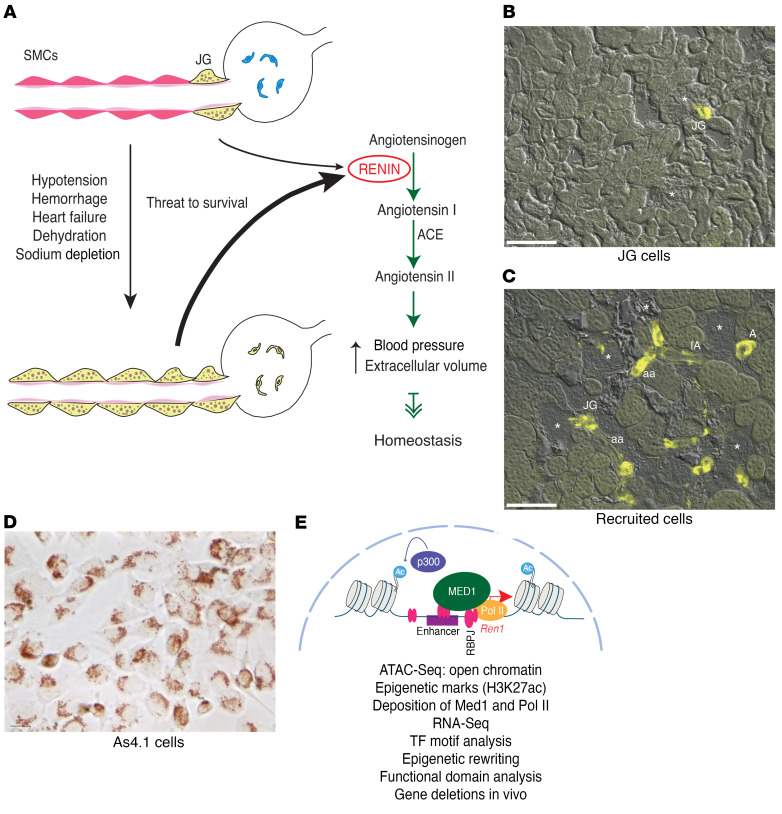
Figure 1. Identity of the renin cells and transformation of renin cell descendants to the renin phenotype when homeostasis is threatened.
The 3 renin cell models used in the present study. (A) Schematic showing juxtaglomerular (JG) cells, the classical renin-expressing cells, located at the entrance to the glomerulus (yellow) in the adult unstressed animal and along the afferent arteriole in response to a threat to survival. These cells secrete the hormone RENIN, a key enzyme of the renin angiotensin system that culminates in the generation of angiotensin II, a powerful vasoconstrictor that regulates blood pressure and fluid electrolyte homeostasis. (B) Kidney section from a Ren1c-YFP WT mouse. In this mouse YFP expression is driven by the renin promoter thus marking the JG renin-expressing cells. The YFP signal is restricted to the afferent arterioles at the entrance to the glomerulus. Scale bar: 100 μM. (C) In renin-deficient mice, SMCs that descended from renin precursors are chronically stimulated to adopt the renin phenotype. Kidney section from a Ren1c–/– Ren1c-YFP mouse. YFP+ cells reporting the activity of the renin promoter are distributed along the afferent arterioles (aa), interlobular arteries (IA), larger intrarenal arteries (A), at the entrance to the glomerulus (JG), and in the mesangium. Scale bar: 100 μM. (D) As4.1 cells, mouse kidney tumoral cells that constitutively produce renin. Numerous renin granules detected with the vital marker neutral red are present in the cytoplasm of these cells. Scale bar: 10 μM. (E) Schematic depicting our working hypotheses and the methods used to test the renin cells using as an example the chromatin at the renin locus. Briefly, we hypothesize that renin cells possess unique sets of renin cell–specific genomic elements such as super-enhancers that determine renin cell identity and the molecular memory of the renin phenotype, allowing renin cell descendants to switch on the renin program when homeostasis is threatened.