Abstract
Primary aldosteronism (PA) is the most common form of secondary hypertension. In many cases, somatic mutations in ion channels and pumps within adrenal cells initiate the pathogenesis of PA, and this mechanism might explain why PA is so common and suggests that milder and evolving forms of PA must exist. Compared with primary hypertension, PA causes more end-organ damage and is associated with excess cardiovascular morbidity, including heart failure, stroke, non-fatal myocardial infarction, and atrial fibrillation. Screening is simple and readily available, and targeted therapy improves blood pressure control and mitigates cardiovascular morbidity. Despite these imperatives, screening rates for PA are low, and mineralocorticoid-receptor antagonists are under-utilized for hypertension treatment. After summarizing the evidence for the prevalence of PA and its associated cardiovascular morbidity, a practical approach to PA screening, referral, and management is described. All physicians who treat hypertension should routinely screen appropriate patients for PA.
Keywords: primary aldosteronism, secondary hypertension, aldosterone, renin, adrenal
A 68-year-old man requested a second opinion from a cardiologist for management of uncontrolled hypertension. Diagnosed with hypertension approximately 35 years prior, he had taken up to 6 antihypertensive medications concurrently without adequate blood pressure control. In the ensuing years, he had developed stage 3 chronic kidney disease. A year prior to consultation, he was admitted to a hospital for new-onset atrial fibrillation in the setting of hypokalemia (serum potassium 2.9 mEq/L). Potassium chloride was initiated at a daily dose of 60 mEq per day. A cardiologist was consulted during that hospitalization, and the medication regimen was adjusted to include a beta-blocker and later clonidine, for a diagnosis of primary hypertension. The patient did not tolerate clonidine. During the second opinion consultation, the patient explained that he was avoiding non-steroidal anti-inflammatory medications, had reduced his alcohol intake, had lost 30 pounds, and was exercising 3-4 times a week. He denied habitually eating confectionery licorice or taking nutritional supplements. He reported excellent adherence with his regimen of hydrochlorothiazide 50 mg daily, lisinopril 60 mg daily, amlodipine 5 mg daily, carvedilol 25 mg twice daily, potassium chloride 60 mEq daily, and apixaban 5 mg daily. Nonetheless, his blood pressure remained uncontrolled (156/89 mmHg). Physical examination revealed 2+ lower extremity edema but no abdominal bruits. Without interruption of his antihypertensive medications, plasma renin activity (PRA) and serum aldosterone concentrations were measured and were 0.2 ng/mL/h (normal range: 0.8-5.3 ng/mL/h upright) and 25.8 ng/dL (inappropriately high for this renin value), respectively. Evaluation for occult secondary causes of hypertension, including thyroid disease and pheochromocytoma, disclosed no other abnormalities. He was referred to an endocrinologist, who confirmed the diagnosis of primary aldosteronism (PA) and completed the evaluation. After laparoscopic adrenalectomy, his ambulatory blood pressure monitoring study showed a 24-hour mean blood pressure of 120 mm Hg systolic and 77 mm Hg diastolic during treatment with lisinopril, hydrochlorothiazide, and carvedilol.
Introduction
PA is defined as inappropriately elevated aldosterone production in the setting of low plasma renin. Once thought to be rare, PA is now known to be the most common cause of secondary hypertension, with a prevalence of approximately 20% among patients with resistant hypertension,1, 2 10% in those with severe hypertension (systolic blood pressure [SBP] ≥180, diastolic BP [DBP] ≥110 mm Hg),3, 4 and 6% in those with otherwise uncomplicated hypertension.4 Only a small fraction of the patients with PA are diagnosed and treated.5 Accumulating evidence suggests that PA amplifies cardiovascular morbidity and mortality beyond primary hypertension, even after controlling for the degree of blood pressure elevation.6-9 Consequently, early identification and specific treatment of PA is essential, yet PA remains under-recognized by both internists and specialists. Screening for PA is simple and accessible and should be routinely implemented in patients with resistant hypertension, hypertension with hypokalemia or early-onset hypertension. The purpose of this review is to highlight a recent explosion of knowledge about PA and to provide a practical approach to its diagnosis and treatment.
A contemporary understanding of PA is essential to the practice of cardiovascular medicine. Funder has estimated that among US patients with PA, 1 in 550 is diagnosed and treated for the condition.5 This conservative estimate illuminates the magnitude of PA underdiagnosis. The recent expansion of knowledge addressing the prevalence, pathophysiology, and clinical approach to PA has provided an evidence-based understanding of this common condition. Although hypokalemia in the setting of hypertension should immediately and reflexively prompt consideration of PA, most patients with PA are not hypokalemic.4, 10, 11 The screening process is much simpler than commonly perceived,12 and the end-organ complications of hypertension due to PA provide an imperative for making the diagnosis and implementing appropriate therapy.13 Although the latter stages of the evaluation are best performed in referral centers,14 all providers with internal medicine training should be comfortable performing initial screening for PA when appropriate and initiating targeted medical therapy.
Aldosterone in normal cardio-renal physiology
Sodium is the primary determinant of plasma osmolarity, and total body sodium is the major determinant of plasma volume. Aldosterone regulates the final stages of sodium reabsorption in the distal renal tubule and collecting duct. Although aldosterone regulates absorption of just 1-5% of filtered sodium, the kidney filters the entire circulating plasma volume twice an hour, totaling approximately 180 liters of plasma a day. The resulting large net daily burden of reabsorption underscores the strategic positioning of aldosterone in regulating plasma volume, as illustrated in the following clinically relevant example. In the setting of a 1,500 mg sodium diet, as recommended in the 2017 ACC/AHA guidelines15 for adults needing blood pressure reduction – equivalent to 0.75 teaspoon of table salt or 65 mEq of sodium – a healthy adult will filter 25,560 mEq (588 g) of sodium16 and reabsorb about 25,553 mEq (99.97% of the filtered load).
Molecular mechanisms of aldosterone effects
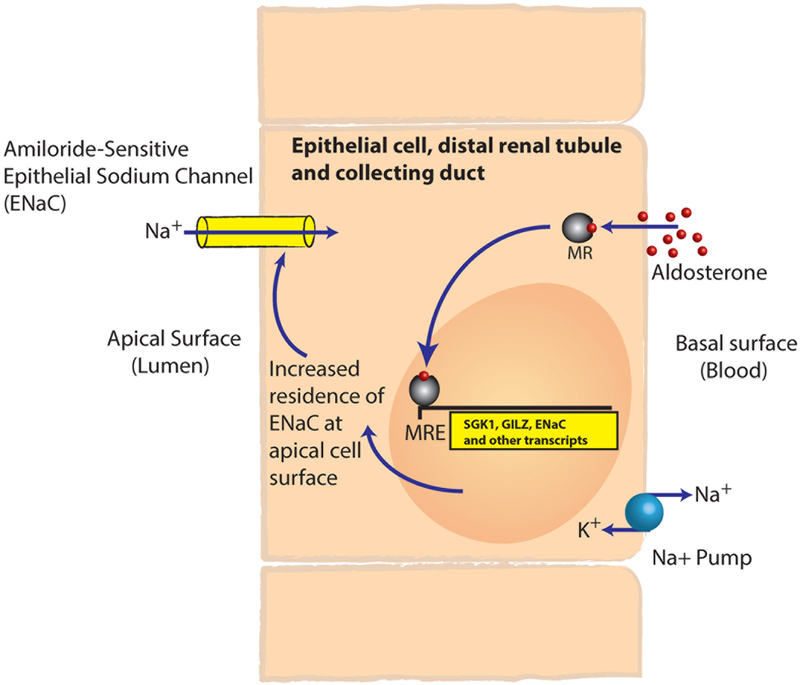
Like other steroid hormones, aldosterone is lipophilic, and half of circulating aldosterone is weakly bound to plasma proteins. Aldosterone diffuses readily through cell membranes, where it binds to the mineralocorticoid receptor (MR), which in the unbound state resides in the cytoplasm. Upon ligand binding, MRs dimerize and translocate to the nucleus, where they act as ligand-activated transcription factors (Figure 1). MR is highly expressed in the epithelial cells lining the distal convoluted tubule and cortical collecting duct of the kidney, the colonic mucosa, and eccrine sweat glands, which are all tissues that transport ions. In addition, MR is expressed in cardiomyocytes, vascular smooth muscle cells, endothelial cells, cells within brown adipose tissue, macrophages, and neurons in several brain regions including the hippocampus, hypothalamus, and brainstem. The consequences of MR activation have been extensively studied in the kidney, where aldosterone promotes the expression of amiloride-sensitive epithelial sodium channels (ENaC) in the distal tubule and cortical collecting duct and their residence on the apical surface, resulting in sodium and water reabsorption. In addition, aldosterone mediates some of its effects in a rapid non-genomic fashion that likely involves MR as well as a receptor called GPER.17-19 Urinary sodium delivered to the distal tubule enters renal tubular epithelial cells (principal cells) via apical ENaC. From the epithelial cells, sodium is reabsorbed back into the interstitial fluid via Na+ /K+-ATPase pumps on the basolateral surface of the cells. As a result of the electrochemical gradient derived from sodium reabsorption, potassium is then secreted into the urine via apical potassium channels. Aldosterone also stimulates the urinary secretion of H+ via the H+-ATPase in the intercalated cells of the cortical collecting tubules. In concert, these renal actions of aldosterone contribute to intravascular volume expansion and renal loss of potassium and hydrogen ions.
Figure 1.
Illustration of the ligand-activated transcription-factor activity of the mineralocorticoid receptor. The liposoluble steroid hormone aldosterone diffuses into cells and binds to and activates the mineralocorticoid receptor. The mineralocorticoid receptor then dimerizes and translocates to the nucleus, where it binds a hormone-response element and regulates the transcription of target genes. This process regulates the abundance of the amiloride-sensitive epithelial-sodium channel on the apical surface of the epithelial cells in the distal-renal tubule and collecting duct, controlling the final 1-5% of sodium reabsorption. MR, mineralocorticoid receptor; MRE, mineralocorticoid response element; SGK1, serum- and glucocorticoid-inducible kinase; GILZ, glucocorticoid-induced leucine zipper; ENaC, amiloride-sensitive epithelial-sodium channel.
Regulation of aldosterone production
In normal physiology, the renin-angiotensin-aldosterone system is the primary regulator of aldosterone production. In the setting of volume depletion or other states of decreased renal perfusion and/or sodium delivery, the juxtaglomerular cells release renin. Renin cleaves angiotensinogen to form angiotensin I, and angiotensin-converting enzyme catalyzes the conversion of angiotensin I to angiotensin II. Angiotensin II receptors on the adrenal zona glomerulosa (ZG) cells signal primarily through depolarization and increased intracellular calcium to induce the enzymatic machinery necessary to synthesize aldosterone, including aldosterone synthase (cytochrome P450 11B2 or CYP11B2).20 Besides angiotensin II, high plasma potassium concentration stimulates and low plasma potassium inhibits aldosterone production; in addition, high potassium potentiates the effect of angiotensin II as an aldosterone secretogogue.21 Adrenocorticotropic hormone (ACTH) transiently stimulates aldosterone synthesis. The net effect of this physiology is that renin and aldosterone normally rise and fall in parallel.
Throughout most of human history, aldosterone protected our interior environment against the threat of salt or volume depletion during stresses, such as salt and water deprivation, high-heat environments, diarrhea, or fever. Historic data from the Yanomamo people of Northeastern Brazil are illustrative of this protective role of aldosterone — and the broad dynamic range over which aldosterone secretion occurs. In the 1970s, the Yanomamo excreted approximately 1 mEq of sodium per day, balancing roughly 1 mEq (23 mg) of daily sodium intake. Their urinary aldosterone levels were 27 times higher and urinary sodium excretion 100-fold lower than members of a research team consuming an ordinary diet that included table salt.22 In contrast, homeostasis of body sodium content and plasma volume requires little or no aldosterone in adults living in developed societies and consuming a high-sodium diet today. Consistent with a reduced need for aldosterone production in societies consuming a high-sodium diet, the adult ZG contains very little aldosterone synthase compared to infants’ ZG.23 Today, the average daily sodium intake in US adults is 3,592 mg, over 4.5 times greater than the 768 mg per day consumed in hunter-gatherer societies. Thus, the modern environment challenges the adult adrenal ZG to minimize aldosterone production, diametrically opposite to sodium preservation, for which it evolved. Consequently, even mild degrees of aldosterone production can be viewed as inappropriate for the high sodium intake in salt-loaded societies and detrimental to health.
Pathogenic mechanisms of PA – How can PA be so common?
Broadly, PA can be dichotomized to unilateral aldosterone production from the right or left adrenal gland (aldosterone-producing adenoma; APA) or bilateral hyperaldosteronism (BHA). In contrast to normal physiology, in PA, the adrenal gland produces aldosterone in an autonomous fashion. Whereas APA is a tangible form of disease, BHA is an elusive concept, which has lacked a pathophysiologic mechanism for many years. The development of highly specific antibodies for aldosterone synthase (CYP11B2) and sophisticated genomic sequencing techniques have led to major advancements in our understanding of PA pathogenesis in the recent years. The simple nomenclature of APA versus BHA derives from basic histology of resected adrenal glands, in conjunction with imaging and adrenal vein sampling. Recent studies utilizing immunohistochemical staining of aldosterone synthase have demonstrated that the PA pathology spans a wide spectrum, that includes bilateral adenomas, unilateral hyperplasia,24, 25 micronodules, and microscopic aldosterone-producing cell clusters (APCCs),26 in various combinations. Importantly, such immunohistochemistry studies have revealed that in unilateral PA, an adrenal gland hosting a macroscopic nodule might contain additional sources of autonomous aldosterone secretion27 and that the largest (“dominant”) nodule is sometimes not a source of aldosterone at all.28, 29
The genesis and progression of PA are not yet fully understood, but several hypotheses have derived from recent studies. In 2010, Nishimoto and colleagues described APCCs as discrete subcapsular cell clusters expressing aldosterone synthase in the normal adrenals,30 in contrast to the continuous ZG observed in rodent adrenals and young human adrenals.31 APCCs were also noted adjacent to APAs,26, 28, 30 despite suppressed renin, suggesting their autonomous aldosterone synthesis. Immunohistochemistry studies of adrenal glands obtained from deceased kidney donors have shown an age-dependent increase in the number of APCCs, in stark contrast with the decline of the total aldosterone synthase-expressing area, further supporting the autonomy of APCCs in aldosterone production.32, 33
The genetic landscape of aldosterone-producing states has yielded further progress in our understanding the molecular mechanisms of PA pathogenesis, starting with studies of familial forms of PA. Three types of familial hyperaldosteronism are currently recognized. Familial hyperaldosteronism type I (FHA-I), also known as glucocorticoid-remediable aldosteronism (GRA), was first described by Sutherland et al. in 196634 and has an autosomal dominant inheritance. In 1992, Lifton and colleagues elucidated the molecular basis of FHA-I, residing in a chimeric gene, which fuses the 5’ end including the promoter of the 11β-hydroxylase gene (CYP11B1) and the 3’ end of the CYP11B2 gene. The enzyme encoded by this hybrid gene produces aldosterone under the regulation of ACTH in the zona fasciculata, where cortisol is normally made. The hypertension of FHA-I tends to be milder than average for PA but is associated with a high incidence of hemorrhagic strokes.
Familial hyperaldosteronism type II (FHA-II) was first described in 1991,35 and is clinically indistinguishable from sporadic PA. While FHA-II appears to be the most common form of inherited PA, the underlying mutation(s) remain unknown, and it is likely a genetically heterogeneous condition. In two FHA-II kindreds, mutations in the CLCN2 chloride channel have been identified.36, 37
Familial hyperaldosteronism type III (FHA-III) was initially reported in 2008, in a family with early-onset, severe PA, and marked bilateral adrenal hyperplasia.38 In 2011, Choi and colleagues revealed the causative germline mutation in this family to be located in the KCNJ5 gene encoding the Kir3.4 (also called GIRK4) potassium channel.39 These mutations alter the selectivity filter of the channel, allowing sodium conductance through what is ordinarily an inward-rectifying potassium channel, which maintains ZG cell hyperpolarization. This change in sodium conductance leads to cell depolarization and calcium entry, which in turn drives aldosterone production.
Germline mutations in CACNA1D (encoding the L-type calcium channel Cav1.3)40 and CACNA1H (encoding the T-type calcium channel Cav3.2)41 have now also been described in families with PA. Many of these cases, which can be considered FHA-IV, have additional neurologic and cognitive dysfunction.
Recent clinical investigation has provided a greater understanding of the pathogenesis of PA and has unexpectedly revealed a high frequency of somatic mutations in genes involved in aldosterone production, which helps to explain the increasing prevalence of PA with age.41-43 Somatic mutations in the same genes causing FHA-III and FHA-IV have been identified in a high proportion of APAs. Choi et al. were the first to report KCNJ5 somatic mutations in APAs,39 findings that several other groups have subsequently confirmed. The percentage of APAs with a mutation in KCNJ5 varies by region, and is as high as 80% in Asian populations.44 Since this discovery, several teams have expanded the spectrum of somatic mutations in APAs that appear to be causally linked to aldosterone production. The somatic mutations in genes encoding other ions channels and pumps include: ATP1A1 (encoding a Na+/K+ ATPase alpha subunit), ATP2B3 (encoding a Ca2+ ATPase), as well as CACNA1D.40, 43, 45,46 In addition, activating somatic mutations of CTNNB1 (β-catenin, an intracellular signal transducer in the Wnt-signaling pathway) have been reported in APAs of pregnant or postmenopausal women, who were found to exhibit dramatic overexpression of ectopic luteinizing hormone/chorionic gonadotropin (LH/hCG) and gonadotropin-releasing hormone (GnRH) receptors, presumably induced by the β-catenin mutations.47 Such aldosterone driver mutations (CACNA1D and ATP1A1) were identified in 8 of 23 (35%) APCCs isolated from kidney donors’ adrenal glands but were not present in the adjacent normal tissue,32 supporting the hypothesis that some APCCs might be precursors of APAs.
Transcriptome analyses have identified several genes whose expression is up-regulated in APAs as compared to normal adjacent tissues, unveiling more possible mechanisms involved in the pathogenesis of PA. Such relevant genes with increased expression in APAs include: enzymes and cofactors required for aldosterone synthesis, including CYP11B2, CYP21A2, adrenodoxin (FDX1), and P450-oxidoreductase (POR);48-51 genes encoding G-protein-coupled receptors, such as LH/hCG receptor (LHR), ACTH receptor (melanocortin receptor type-2, MC2R), GnRH receptor (GNRHR), and serotonin receptor 4 (HTR4);52 genes encoding transcription factors involved in steroidogenesis and differentiation;49, 51 and calmodulin kinase (CAMK), encoding a protein involved in Ca2+ signaling.50 All of these proteins enhance the steroidogenic capacity of adrenal cells.
Despite this increasingly detailed understanding of APAs, the origins of BHA remain poorly understood. One possibility is that BHA derives from the accumulation of a clinically significant number of APCCs in both adrenal glands. In mice, deletion of the TWIK-related acid-sensitive potassium channels types 1 and 3 (TASK-1, TASK-3) causes bilateral PA.53 TASK-2 expression is decreased in some adrenal nodules from patients with PA;54 however, the significance of altered TASK function as an etiologic factor in BHA remains speculative. Whereas in unilateral PA adrenal glands are often removed to treat APAs, surgery is rarely used for BHA, which limits access to BHA tissue samples and represents a comparative challenge for molecular studies of BHA pathogenesis.
Importantly, PA is not a yes/no dichotomy in hypertensive patients but rather a spectrum of MR antagonist-responsive disease, particularly in patients with resistant hypertension and mildly elevated or even ostensibly normal aldosterone levels. Calhoun and colleagues have explored this phenotype extensively,55 and Vaidya and colleagues have further characterized this continuum using comprehensive dynamic testing.56 Additional evidence for this mild PA phenotype comes from the PATHWAY-2 trial. Patients with resistant hypertension often responded well to MR antagonists in PATHWAY-2, despite the exclusion of patients with classical PA from the study and irrespective of baseline aldosterone levels.57 It is not known whether MR-responsive hypertension in the setting of ostensibly normal circulating aldosterone indicates a relative excess of aldosterone or hypersensitivity to aldosterone, MR activation via occult ligands, or other mechanisms.
Clinical Consequences of Primary Aldosteronism
PA due to an APA is called Conn Syndrome. The German Conn Syndrome Registry, which includes all forms of PA, is a multicenter registry intended to study PA with adequate statistical power despite low screening rates. In the German Conn Syndrome Registry, overall mortality was higher in patients with PA compared to non-hypertensive controls but not different from patients with primary hypertension. Death from cardiovascular causes was more common among patients with PA compared to matched controls with primary hypertension.58
Fibrosis of the heart, adrenal glands, pancreas, and lungs has been found at autopsy in patients with PA.59 Although PA is most often detected in patients with hypertension—who are more likely to be screened—PA likely affects the cardiovascular system even in normotensive individuals with early and mild PA. Stowasser and colleagues studied a small number of normotensive patients with familial forms of PA, who were found to have concentric left ventricular hypertrophy and poorer diastolic function compared to age- and sex-matched normotensive controls.7 Freel, et al.60 found that patients with PA more often have a diffuse, non-infarct pattern of late gadolinium enhancement on cardiac MRI compared to control study participants with primary hypertension – a finding that was independent of blood pressure. In addition, patients with PA more often have exercise-induced myocardial ischemic defects on SPECT and echocardiography compared to patients with primary hypertension.61 This evidence of myocardial fibrosis is concordant with animal studies of secondary hyperaldosteronism in the setting of high-salt diet.62, 63 In addition, to the extent that myocardial fibrosis contributes to arrhythmias and sudden death in heart failure, these imaging data are consistent with the observation that spironolactone or eplerenone improves mortality in heart failure (e.g., the RALES and EPHESUS trials).64, 65 More than simply affecting the heart and kidneys, PA is associated with vascular remodeling characterized by a marked increase in media:lumen ratio.66
These small-scale studies of intermediate phenotypes have led to larger studies of health outcomes in PA. Milliez, et al. found that compared to patients with primary hypertension, patients with PA were more likely to have experienced stroke, non-fatal myocardial infarction, or atrial fibrillation (odds ratios of 4.2 [95% confidence interval [CI] 2.0 – 8.6, 6.5 [95% CI 1.5 to 27.4], and 12.1 [95% CI 3.2 to 45.2], respectively).6 These findings were also independent of blood pressure. Hence, the man in our vignette illustrates several common consequences of long-standing PA, manifesting in his case with atrial fibrillation and hypokalemia after years of uncontrolled hypertension. To avoid the enrichment for unusual diseases expected in specialty referral centers, Monticone, et al. prospectively screened for PA in unselected patients with hypertension recruited from primary care clinics.4 Metabolic syndrome was more common among the 5.9% of patients diagnosed with PA compared to other hypertensive patients, consistent with earlier studies suggesting an effect of PA on metabolism.67 A meta-analysis of 3,838 patients with PA compared with 9,284 patients with primary hypertension also demonstrated adverse cardiovascular outcomes in PA.68
Diagnosis of PA
While the later stages of the evaluation and subtyping are commonly deferred to endocrinologists or other skilled hypertension specialists at referral centers, screening for PA is simple, widely available, and relatively inexpensive. Despite this, PA remains under-recognized, and the diagnosis is often delayed for many years. In view of the cardio-renal and cerebrovascular implications of PA when left untreated, it is imperative to suspect and to screen for PA early. Thus, all providers treating hypertension, including primary care practitioners, cardiologists, and nephrologists, should routinely perform PA screening in appropriate patients.
Screening for PA
The 2017 ACC/AHA hypertension guidelines recommend screening for PA in high-risk populations including patients with: 1) resistant hypertension; 2) hypertension and spontaneous or diuretic-induced hypokalemia; 3) hypertension and an adrenal mass; 4) hypertension and a family history of early onset hypertension or cerebrovascular accident at a young age (<40 years).15 These guidelines have revised the definition of resistant hypertension to be an office SBP/DBP ≥130/80 mm Hg and prescription of ≥3 antihypertensive medications at optimal doses, including a diuretic if possible, or an office SBP/DBP <130/80 mm Hg for a patient requiring ≥4 antihypertensive medications. The Endocrine Society clinical practice guidelines include a few additional high-risk populations for PA (Table 1).12 For example, obstructive sleep apnea is common in patients with resistant hypertension, and this population has a high prevalence of PA.69, 70 Note that all of these recommendations were based on studies conducted prior to the introduction of the lower threshold for hypertension in the new ACC/AHA 2017 guidelines.
Table 1.
Indications for PA screening
| Hypertension resistant to three conventional antihypertensive drugs |
| Hypertension and hypokalemia (spontaneous or diuretic-induced) |
| Hypertension and a family history of early onset hypertension or cerebrovascular accident at a young age (<40 years) |
| Hypertension and an adrenal tumor |
| Controlled BP on four or more antihypertensive drugs |
| Sustained BP above 150/100 mm Hg, measured on 3 different days |
| Hypertension and sleep apnea |
| All hypertensive first-degree relatives of patients with PA |
Serum (or plasma) aldosterone and plasma renin are utilized in PA screening with a simple blood draw. The aldosterone-to-renin ratio (ARR) has been widely recommended as a screen for PA, but we suggest first identifying a low renin (PRA <1 ng/mL/h) and then requiring an aldosterone >10 ng/dL, for the following reasons.71 When PRA is used, the ARR (aldosterone in ng/dL and renin in ng/mL/h) in the population is not normally distributed, having a median of about 5 and few values >10.72, 73 An ARR >20, which is commonly used as the threshold for positive PA screening, had a sensitivity of 78% and a specificity of 83% in study participants with resistant hypertension.74 The most important pitfall of PA screening is that some laboratories report PRA values to a lower limit of 0.1 ng/mL/h. In this circumstance, the ARR is disproportionately influenced by its denominator75 and can meet the threshold of 20 even when aldosterone is as low as 2 ng/dL – these values are not diagnostic of PA. Thus, caution should be used in interpreting the ARR when PRA is reported to values <0.6 ng/mL/h. Another potential pitfall in using the ARR is that many clinical laboratories are transitioning to replacing PRA with DRC, which yields different ARR values. The DRC in pg/mL is often roughly a factor of 10 higher and has a much wider linear dynamic range at high values.
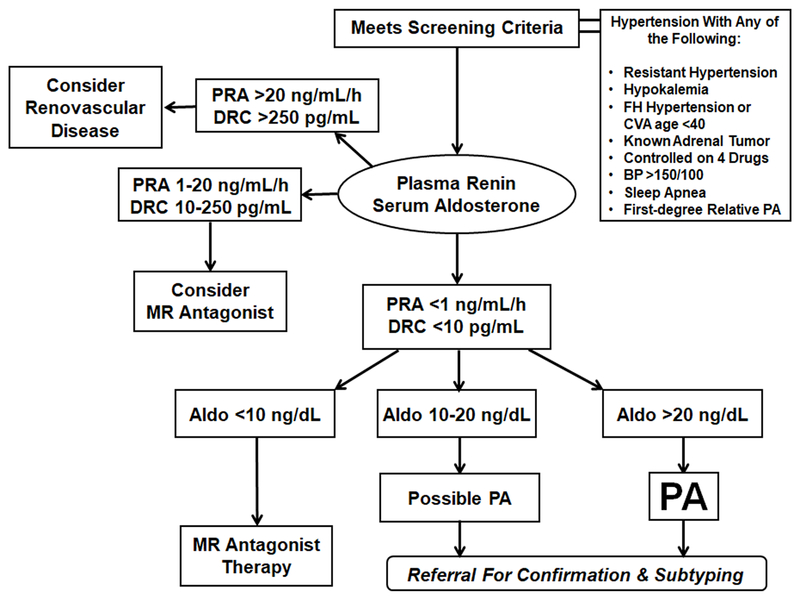
Because of these potential pitfalls in interpreting the ARR, we recommend the following straightforward approach to interpreting the aldosterone and the renin when screening for PA. Our approach is based on the logic of the ARR but is designed to guard against false positives caused by very low renin measurements, while maintaining simplicity. Clinically, a PRA <1 ng/mL/h or DRC <10 pg/mL is considered suppressed and indicative of volume expansion, and a simultaneous aldosterone >10 ng/dL raises suspicion of PA. As previously mentioned, hypokalemia inhibits aldosterone production, so an even lower aldosterone could be present in hypokalemic PA. For that reason, correction of hypokalemia to a plasma K+ of 4.0 prior to testing is recommended. A practical approach to PA screening and triaging for patients with inadequate hypertension control is shown in Figure 2.
Figure 2.
Simplified algorithm for PA screening and triaging based primarily on plasma renin and secondarily on serum aldosterone. Aldo, aldosterone; BP, blood pressure; CVA, cerebrovascular accident; DRC, direct renin concentration; FH, family history; MR, mineralocorticoid receptor; PA, primary aldosteronism; PRA, plasma renin activity. Patients with low renin and aldosterone >10 ng/dL are referred for confirmatory testing and subtyping; most patients with aldosterone >20 ng/dL have PA, and those with hypokalemia do not require confirmatory testing.
Some technical aspects of the assays used to diagnose PA are worth knowing. Blood samples for measurement of aldosterone and renin (either PRA or direct renin concentration [DRC]) are ideally collected simultaneously in the morning, after patients have been ambulatory for at least 2 hours. While several factors can influence aldosterone and renin results, aldosterone and renin still rise or fall concurrently in patients without PA. Most antihypertensive drugs except beta blockers tend to raise renin and aldosterone, although not proportionately. When using the ARR, false positives occur in 14% to 22% of screening tests in resistant hypertension patients, depending upon which antihypertensive medications are used.74 To avoid false-positives, we recommend that positive PA screens are defined as those with both low renin and high aldosterone, rather than a specific ARR.71 Table 2 lists the major causes of false-negatives and false-positives for PA screening.
Table 2.
Major sources of error in PA screening.
| Factor | Mechanism |
|---|---|
| False positives | |
| Hyperkalemia | Directly stimulates aldosterone production |
| Calculating aldosterone/renin ratio | Artificially inflates ratio with renin values <0.6 ng/mL/h |
| Direct renin inhibitors | Lowers plasma renin activity |
| Oral contraceptives or estrogen | Lowers direct renin concentration |
| False negatives | |
| Hypokalemia | Impairs aldosterone production |
| MR antagonists | Raise renin in patients with PA and can increase aldosterone |
| Angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers | Increase renin disproportionate to aldosterone |
| Diuretics & sodium restriction | Rarely raise renin in patients with PA |
| Pregnancy | Disproportionately raises renin, especially plasma renin activity |
Discontinuation of antihypertensive medications to facilitate testing for PA has some risks. Thus, upon first of suspicion of PA, we recommend screening patients in the office without discontinuing medications. Patients with PA demonstrate persistent volume expansion and resistance to typical antihypertensive medications, and screening results can still be interpreted as long as renin is suppressed.76 In fact, emerging evidence suggests that even the latter stages of the evaluation can be performed successfully if renin remains suppressed despite MR antagonist therapy.77 Although mild cases might be missed when screening during medication treatment, most patients with significant PA who will maximally benefit from further evaluation will still have suppressed renin (i.e., PRA <1 ng/mL/h or DRC <10 pg/mL). When renin is not suppressed but the ARR and index of suspicion are high, interfering medications should be reduced or discontinued, and antihypertensive agents with no or minimal interference with the RAAS substituted, such as α-adrenergic blockers, hydralazine and verapamil.12 In such an instance, screening with aldosterone and renin measurement should be repeated after 2 or more weeks on the new regimen. Prior to repeat testing, serum potassium should be normalized, and dietary sodium should be high.78
Confirmatory testing for PA
Patients who screen positive for PA should be referred to an endocrinologist or hypertension specialist for confirmatory testing and subtyping. To confirm the diagnosis of PA, most centers in the United States use salt loading tests to induce volume expansion, which suppresses aldosterone production in normal individuals but not in patients with PA, in whom the aldosterone secretion is autonomous. The 24 h urine aldosterone measurement is collected on the third day of oral salt loading; alternatively, a serum aldosterone is collected following intravenous saline infusion of 2 L over 4 h. Additional options include the fludrocortisone suppression test and captopril challenge test, which are less standardized, more difficult to perform safely (fludrocortisone suppression test) and prone to equivocal results (captopril challenge test). Patients with spontaneous hypokalemia, suppressed renin, and plasma aldosterone concentration >20 ng/dL are diagnosed with unequivocal PA from screening alone (Figure 2).12
Subtyping of PA
Unilateral disease is most often due to an aldosterone- APA (typically <2 cm), rarely a carcinoma (often >6 cm), and occasionally larger (2-4 cm) adenomas that co-produce aldosterone and cortisol. Laparoscopic adrenalectomy is highly successful for treating APA with complete biochemical success in 94% of patients and about 85% partial or complete clinical success at specialized centers.79 In contrast, unilateral adrenalectomy is about half as successful for BHA as for APA,80 and surgical removal of both adrenal glands is not recommended for BHA due to the resultant morbid state of adrenal insufficiency. Once the diagnosis of PA is established, the patient should be counseled about the potential for surgical remediation with adrenalectomy for unilateral disease. If the patient is not a surgical candidate or unwilling to pursue further evaluation and surgery, medical therapy with MR antagonist (spironolactone or eplerenone) is the treatment of choice. If the patient is a surgical candidate and wishes to pursue the necessary pre-operative evaluation, additional testing known as subtyping is necessary.
Current guidelines for the workup of PA recommend that all patients with PA should undergo adrenal imaging to rule out adrenal carcinoma. Adrenal imaging is inaccurate in determining which adrenal gland(s) are the source(s) of PA (i.e., “laterality”) because small adrenal adenomas are common, and imaging features cannot distinguish APAs from non-functional tumors. The test of choice for PA subtyping is adrenal vein sampling, not cross-sectional imaging.12 After the diagnosis of PA is confirmed and adrenal imaging has been done, adrenal vein sampling should be performed in patients who would be interested in adrenalectomy to ameliorate their PA if the source is determined to be one (not both) adrenal gland. The discussion of whether to undertake adrenal vein sampling should be in consultation with an endocrinologist or hypertension expert who can delineate the risks and benefits of laparoscopic adrenalectomy. Adrenal vein sampling is technically challenging and should be performed in a tertiary referral center, by an experienced interventional radiologist using consistent protocols and interpretation criteria.81
Medical therapy for PA – Pearls and pitfalls
If the patient is not a surgical candidate or has BHA, medical therapy with spironolactone or eplerenone is indicated. Spironolactone is effective for treating PA in most patients, inexpensive, and widely available, but due to some uncertainties regarding long term outcomes with MRA therapy, surgery remains the treatment or choice for appropriate candidates. Spironolactone has a slow onset of action relative to vasodilators. Thus, a low dose (12.5-25 mg once daily) added to current antihypertensive medications is a good starting dose of spironolactone for PA. Electrolytes, creatinine, and blood pressure are reassessed in 4-8 weeks (before titrating the dose upward), but these tests must be assessed sooner in patients with renal insufficiency, who are prone to develop hyperkalemia with MR antagonists. Because PA induces a hyperfiltration state, a small rise in serum creatinine is expected in PA patients after surgical cure or with MR antagonist therapy, reflecting effects on glomerular hemodynamics and not glomerular injury. The daily spironolactone dose is typically increased by 25-50 mg every 4-8 weeks until the patient maintains a serum potassium in the upper half of normal without supplements. Spironolactone can be divided twice daily once a total daily dose of 100 mg or more is reached. Non-adherence and incomplete adherence are common in resistant hypertension, including patients ultimately found to PA.82 Consideration should be given to therapeutic drug monitoring before advancing the dose,83 particularly when hypokalemia remains uncorrected. The literature describes spironolactone doses of up to 200 mg daily for PA.84 We are aware of anecdotal reports of 200-400 mg daily doses used chronically for PA. In studies outside the setting of PA, daily doses as high as 500 mg have been used.85
One study of 602 PA patients treated medically found that the adverse cardiovascular outcomes are mitigated if the MR antagonist dose is titrated to also raise PRA to >1 ng/mL/h.86 Some authorities monitor PRA and titrate the spironolactone dose to a normal PRA. If in the judgment of the clinician the blood pressure falls too far below the goal of 130/80 mm Hg during titration, other antihypertensive medications are discontinued based on side effect profiles and mechanisms of action. If the blood pressure remains elevated after potassium is normalized or the maximum tolerated dose is reached, additional antihypertensive medications are adjusted to achieve target blood pressure.
Common side effects of spironolactone in menstruating women include spotting between menses and breast tenderness at high doses. Spironolactone should be combined with contraception for women of reproductive potential, due to potential teratogenic effects on a male fetus. Men can develop gynecomastia and sexual dysfunction due to spironolactone’s antagonism of testosterone, but this effect is dose-dependent and takes weeks to months to develop – usually well after blood pressure has nadired. The incidence of gynecomastia may be as high as 30% at 100 mg daily and 62% at 200 mg daily.87 If adverse effects of spironolactone develop, eplerenone is substituted, usually given twice daily at a dose twice that of spironolactone – for example, 50 mg eplerenone twice daily exchanged for 50 mg spironolactone once daily. Eplerenone is generally more expensive than spironolactone and can be considered initial MRA therapy if cost is not prohibitive. The principles of titrating eplerenone dosing are the same as for spironolactone. Published studies of eplerenone for hypertension used doses up to 400 mg per day, despite the package insert limiting doses to 50 mg twice daily, reflecting concerns over hyperkalemia in patients with heart failure and renal insufficiency.88, 89 The most common mistakes made in prescribing spironolactone for PA are starting at too high a dose and/or titrating up too quickly. The most common mistake in prescribing eplerenone for PA is not using a high enough dose and not dividing the dose twice daily.
Studies of proteinuria and left ventricular hypertrophy regression in patients with PA have shown similar improvements in patients treated surgically or medically. While these findings might be reassuring for patients managed with an MR antagonist, some uncertainty remains in how to adequately titrate medical therapy. Once blood pressure and serum potassium are normal, can one assume that all MR in the heart, kidney, brain, and vasculature is also adequately antagonized? Can residual aldosterone have detrimental MR-independent effects? What about low amounts of autonomous cortisol co-secretion from smaller tumors? Some unsettling data regarding these concerns have begun to appear. One large prospective study of PA patients treated medically or surgically found higher cardiovascular mortality among those treated medically, with the caveats that patients were not randomized and that surgery might not be offered to patients with poor prognosis.8 A study from Taiwan found evidence of glucose tolerance deterioration in PA patients treated medically,90 possibly reflecting failure to address glucocorticoid production from some of these tumors.91 Until more data from prospective studies are available, medical therapy remains a good option, but surgical therapy might have advantages, in addition to being more cost-effective in young patients.92
What about patients who screen negative for PA?
The prevalence of PA in patients with resistant hypertension might be as high as 20%, but then 80% of that group will screen negative for PA. Some of these patients might have a mild form of PA, not meeting criteria for a formal diagnosis yet possibly contributing to their hypertension.93 Some of these patients might progress to overt PA over time, but such prospective studies have not been conducted. Regardless, patients with resistant hypertension respond well to spironolactone, with placebo-adjusted reductions in ambulatory systolic BP averaging 5.4 mm Hg94 and placebo-adjusted home systolic BP reduction averaging 8.7 mm Hg.57 The PATHWAY-2 study demonstrated the superiority of spironolactone to bisoprolol or doxazosin added to existing regimens in patients with resistant hypertension, except in patients with the highest DRC.57 Close monitoring of electrolytes is essential when using MR antagonists. Therefore, we recommend treatment with an MR antagonist for those resistant hypertension patients who meet criteria for PA screening and whose renin is normal or low but whose aldosterone is not high enough for a positive PA screen.
Future Directions
Some major causes of clinical inertia and impediments to screening for PA include the complexity and cost of the latter stages of the evaluation. Although some data support the cost-effectiveness95 of the diagnostic process we have suggested above, approaches to reduce the cost of PA evaluation are anticipated. The majority of PA cases identified through expanded screening are patients with BHA, who ultimately require MR antagonist therapy, yet still often undergo CT imaging and adrenal vein sampling without finding a surgical target. To streamline the evaluation, noninvasive strategies for PA subtyping have been recently proposed. Steroid profiling of adrenal vein and peripheral serum samples from PA patients has led to the identification of biomarkers with potential to discriminate APAs from BHA. For example, peripheral plasma concentrations of 18-oxocortisol, a “hybrid steroid” (bearing both aldosterone and cortisol functionalities), are higher in patients with APA than BHA, while those of cortisol, corticosterone, and dehydroepiandrosterone (DHEA) are lower.96-98 The utility of peripheral 18-oxocortisol and 18-hydroxycortisol to discriminate between APA and BHA is particularly high in Japanese populations, where KCNJ5 mutations in APAs are frequent.96 The targeted implementation of these evolving diagnostic tools is likely to reduce the need for adrenal vein sampling in patients with BHA, reduce cost of the evaluation, and encourage broader screening for PA in populations with a high prevalence of this common form of hypertension, which is highly amenable to targeted therapy or even cure.
Acknowledgments
Sources of Funding: JBB was supported by grant 5K23HL128909; AFT was supported by grant 1K08DK109116; RJA was supported by grant R21DK103183, all from the National Institutes of Health.
Footnotes
Disclosures: Nothing to disclose
References
- 1.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB. and Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892-896. [DOI] [PubMed] [Google Scholar]
- 2.Strauch B, Zelinka T, Hampf M, Bernhardt R. and Widimsky J., Jr. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J Hum Hypertens 2003;17:349-352. [DOI] [PubMed] [Google Scholar]
- 3.Mosso L, Carvajal C, Gonzalez A, Barraza A, Avila F, Montero J, Huete A, Gederlini A. and Fardella CE. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42:161-165. [DOI] [PubMed] [Google Scholar]
- 4.Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F. and Mulatero P. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol 2017;69:1811-1820. [DOI] [PubMed] [Google Scholar]
- 5.Funder JW. Mineralocorticoid receptor antagonists: emerging roles in cardiovascular medicine. Integr Blood Press Control. 2013;6:129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME. and Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 2005;45:1243-1248. [DOI] [PubMed] [Google Scholar]
- 7.Stowasser M, Sharman J, Leano R, Gordon RD, Ward G, Cowley D. and Marwick TH. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. J Clin Endocrinol Metab 2005;90:5070-5076. [DOI] [PubMed] [Google Scholar]
- 8.Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R. and Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med 2008;168:80-85. [DOI] [PubMed] [Google Scholar]
- 9.Yoshihara F, Nishikimi T, Yoshitomi Y, Nakasone I, Abe H, Matsuoka H. and Omae T. Left ventricular structural and functional characteristics in patients with renovascular hypertension, primary aldosteronism and essential hypertension. Am J Hypertens 1996;9:523-528. [DOI] [PubMed] [Google Scholar]
- 10.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC. and Mantero F. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol 2006;48:2293-2300. [DOI] [PubMed] [Google Scholar]
- 11.Mulatero P. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 2004;89:1045-1050. [DOI] [PubMed] [Google Scholar]
- 12.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M. and Young WF., Jr. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016; 101:1889-1916. [DOI] [PubMed] [Google Scholar]
- 13.Savard S, Amar L, Plouin PF. and Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331-336. [DOI] [PubMed] [Google Scholar]
- 14.Rossi GP, Barisa M, Allolio B, Auchus RJ, Amar L, Cohen D, Degenhart C, Deinum J, Fischer E, Gordon R, Kickuth R, Kline G, Lacroix A, Magill S, Miotto D, Naruse M, Nishikawa T, Omura M, Pimenta E, Plouin PF, Quinkler M, Reincke M, Rossi E, Rump LC, Satoh F, Schultze Kool L, Seccia TM, Stowasser M, Tanabe A, Trerotola S, Vonend O, Widimsky J, Jr., Wu KD, Wu VC. and Pessina AC. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab 2012;97:1606-1614. [DOI] [PubMed] [Google Scholar]
- 15.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Sr., Williamson JD. and Wright JT., Jr. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269-1324. [DOI] [PubMed] [Google Scholar]
- 16.National Research Council Sodium-restricted diets: the rationale, complications, and practical aspects of their use. Washington: Division of Biology and Agriculture, National Research Council; 1954. [Google Scholar]
- 17.Ong GS. and Young MJ. Mineralocorticoid regulation of cell function: the role of rapid signalling and gene transcription pathways. J Mol Endocrinol 2017;58:R33-R57. [DOI] [PubMed] [Google Scholar]
- 18.Feldman RD. and Limbird LE. GPER (GPR30): A Nongenomic Receptor (GPCR) for Steroid Hormones with Implications for Cardiovascular Disease and Cancer. Annu Rev Pharmacol Toxicol 2017;57:567-584. [DOI] [PubMed] [Google Scholar]
- 19.Coutinho P, Vega C, Pojoga LH, Rivera A, Prado GN, Yao TM, Adler G, Torres-Grajales M, Maldonado ER, Ramos-Rivera A, Williams JS, Williams G. and Romero JR. Aldosterone's rapid, nongenomic effects are mediated by striatin: a modulator of aldosterone's effect on estrogen action. Endocrinology. 2014;155:2233-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carithers LJ, Ardlie K, Barcus M, Branton PA, Britton A, Buia SA, Compton CC, DeLuca DS, Peter-Demchok J, Gelfand ET, Guan P, Korzeniewski GE, Lockhart NC, Rabiner CA, Rao AK, Robinson KL, Roche NV, Sawyer SJ, Segre AV, Shive CE, Smith AM, Sobin LH, Undale AH, Valentino KM, Vaught J, Young TR. and Moore HM. A Novel Approach to High-Quality Postmortem Tissue Procurement: The GTEx Project. Biopreserv Biobank. 2015;13:311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young DB, Smith MJ, Jr., Jackson TE. and Scott RE. Multiplicative interaction between angiotensin II and K concentration in stimulation of aldosterone. Am J Physiol 1984;247:E328-E335. [DOI] [PubMed] [Google Scholar]
- 22.Oliver WJ, Cohen EL. and Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a "no-salt" culture. Circulation. 1975;52:146-151. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H. and Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol 2014;383:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iacobone M, Citton M, Viel G, Boetto R, Bonadio I, Tropea S, Mantero F, Rossi GP, Fassina A, Nitti D. and Favia G. Unilateral adrenal hyperplasia: a novel cause of surgically correctable primary hyperaldosteronism. Surgery. 2012;152:1248-1255. [DOI] [PubMed] [Google Scholar]
- 25.Shigematsu K, Yamaguchi N, Nakagaki T. and Sakai H. A case of unilateral adrenal hyperplasia being difficult to distinguish from aldosterone-producing adenoma. Exp Clin Endocrinol Diabetes. 2009;117:124-128. [DOI] [PubMed] [Google Scholar]
- 26.Nanba K, Tsuiki M, Sawai K, Mukai K, Nishimoto K, Usui T, Tagami T, Okuno H, Yamamoto T, Shimatsu A, Katabami T, Okumura A, Kawa G, Tanabe A. and Naruse M. Histopathological diagnosis of primary aldosteronism using CYP11B2 immunohistochemistry. J Clin Endocrinol Metab 2013;98:1567-1574. [DOI] [PubMed] [Google Scholar]
- 27.Dekkers T, ter Meer M, Lenders JW, Hermus AR, Schultze Kool L, Langenhuijsen JF, Nishimoto K, Ogishima T, Mukai K, Azizan EA, Tops B, Deinum J. and Kusters B. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J Clin Endocrinol Metab 2014;99:E1341-E1351. [DOI] [PubMed] [Google Scholar]
- 28.Monticone S, Castellano I, Versace K, Lucatello B, Veglio F, Gomez-Sanchez CE, Williams TA. and Mulatero P. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol 2015;411:146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nanba AT, Nanba K, Byrd JB, Shields JJ, Giordano TJ, Miller BS, Rainey WE, Auchus RJ. and Turcu AF. Discordance between imaging and immunohistochemistry in unilateral primary aldosteronism. Clin Endocrinol (Oxf) 2017;87:665-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M. and Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab 2010;95:2296-2305. [DOI] [PubMed] [Google Scholar]
- 31.Nishimoto K, Seki T, Hayashi Y, Mikami S, Al-Eyd G, Nakagawa K, Morita S, Kosaka T, Oya M, Mitani F, Suematsu M, Kabe Y. and Mukai K. Human Adrenocortical Remodeling Leading to Aldosterone-Producing Cell Cluster Generation. Int J Endocrinol 2016;2016:7834356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimoto K, Tomlins SA, Kuick R, Cani AK, Giordano TJ, Hovelson DH, Liu CJ, Sanjanwala AR, Edwards MA, Gomez-Sanchez CE, Nanba K. and Rainey WE. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A 2015;112:E4591-E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanba K, Vaidya A, Williams GH, Zheng I, Else T. and Rainey WE. Age-Related Autonomous Aldosteronism. Circulation. 2017;136:347-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutherland DJ, Ruse JL. and Laidlaw JC. Hypertension, increased aldosterone secretion and low plasma renin activity relieved by dexamethasone. Can Med Assoc J 1966;95:1109-1119. [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon RD, Stowasser M, Tunny TJ, Klemm SA, Finn WL. and Krek AL. Clinical and pathological diversity of primary aldosteronism, including a new familial variety. Clin Exp Pharmacol Physiol 1991;18:283-286. [DOI] [PubMed] [Google Scholar]
- 36.Scholl UI, Stolting G, Schewe J, Thiel A, Tan H, Nelson-Williams C, Vichot AA, Jin SC, Loring E, Untiet V, Yoo T, Choi J, Xu S, Wu A, Kirchner M, Mertins P, Rump LC, Onder AM, Gamble C, McKenney D, Lash RW, Jones DP, Chune G, Gagliardi P, Choi M, Gordon R, Stowasser M, Fahlke C. and Lifton RP. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat Genet 2018;50:349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandes-Rosa FL, Daniil G, Orozco IJ, Goppner C, El Zein R, Jain V, Boulkroun S, Jeunemaitre X, Amar L, Lefebvre H, Schwarzmayr T, Strom TM, Jentsch TJ. and Zennaro MC. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat Genet 2018;50:355-361. [DOI] [PubMed] [Google Scholar]
- 38.Geller DS, Zhang J, Wisgerhof MV, Shackleton C, Kashgarian M. and Lifton RP. A novel form of human mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab 2008;93:3117-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Akerstrom G, Wang W, Carling T. and Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Akerstrom G, Bjorklund P, Carling T, Fahlke C, Hidalgo P. and Lifton RP. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 2013;45:1050-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scholl UI, Stolting G, Nelson-Williams C, Vichot AA, Choi M, Loring E, Prasad ML, Goh G, Carling T, Juhlin CC, Quack I, Rump LC, Thiel A, Lande M, Frazier BG, Rasoulpour M, Bowlin DL, Sethna CB, Trachtman H, Fahlke C. and Lifton RP. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. eLife. 2015;4:e06315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scholl UI, Goh G, Stolting G, de Oliveira RC, Choi M, Overton JD, Fonseca AL, Korah R, Starker LF, Kunstman JW, Prasad ML, Hartung EA, Mauras N, Benson MR, Brady T, Shapiro JR, Loring E, Nelson-Williams C, Libutti SK, Mane S, Hellman P, Westin G, Akerstrom G, Bjorklund P, Carling T, Fahlke C, Hidalgo P. and Lifton RP. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet 2013;45:1050-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, Annaratone L, Castellano I, Beuschlein F, Reincke M, Lucatello B, Ronconi V, Fallo F, Bernini G, Maccario M, Giacchetti G, Veglio F, Warth R, Vilsen B. and Mulatero P. Somatic ATP1A1, ATP2B3, and KCNJ5 Mutations in Aldosterone-Producing Adenomas. Hypertension. 2014;63:188-195. [DOI] [PubMed] [Google Scholar]
- 44.Taguchi R, Yamada M, Nakajima Y, Satoh T, Hashimoto K, Shibusawa N, Ozawa A, Okada S, Rokutanda N, Takata D, Koibuchi Y, Horiguchi J, Oyama T, Takeyoshi I. and Mori M. Expression and mutations of KCNJ5 mRNA in Japanese patients with aldosterone-producing adenomas. J Clin Endocrinol Metab 2012;97:1311-1319. [DOI] [PubMed] [Google Scholar]
- 45.Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM. and Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet 2013;45:440-444, 444e441-442. [DOI] [PubMed] [Google Scholar]
- 46.Azizan EA, Poulsen H, Tuluc P, Zhou J, Clausen MV, Lieb A, Maniero C, Garg S, Bochukova EG, Zhao W, Shaikh LH, Brighton CA, Teo AE, Davenport AP, Dekkers T, Tops B, Kusters B, Ceral J, Yeo GS, Neogi SG, McFarlane I, Rosenfeld N, Marass F, Hadfield J, Margas W, Chaggar K, Solar M, Deinum J, Dolphin AC, Farooqi IS, Striessnig J, Nissen P. and Brown MJ. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat Genet 2013;45:1055-1060. [DOI] [PubMed] [Google Scholar]
- 47.Teo AE, Garg S, Shaikh LH, Zhou J, Karet Frankl FE, Gurnell M, Happerfield L, Marker A, Bienz M, Azizan EA. and Brown MJ. Pregnancy, Primary Aldosteronism, and Adrenal CTNNB1 Mutations. N Engl J Med 2015;373:1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Assie G, Auzan C, Gasc JM, Baviera E, Balaton A, Elalouf JM, Jeunemaitre X, Plouin PF, Corvol P. and Clauser E. Steroidogenesis in aldosterone-producing adenoma revisited by transcriptome analysis. J Clin Endocrinol Metab 2005;90:6638-6649. [DOI] [PubMed] [Google Scholar]
- 49.Bassett MH, Mayhew B, Rehman K, White PC, Mantero F, Arnaldi G, Stewart PM, Bujalska I. and Rainey WE. Expression profiles for steroidogenic enzymes in adrenocortical disease. J Clin Endocrinol Metab 2005;90:5446-5455. [DOI] [PubMed] [Google Scholar]
- 50.Lenzini L, Seccia TM, Aldighieri E, Belloni AS, Bernante P, Giuliani L, Nussdorfer GG, Pessina AC. and Rossi GP. Heterogeneity of aldosterone-producing adenomas revealed by a whole transcriptome analysis. Hypertension. 2007;50:1106-1113. [DOI] [PubMed] [Google Scholar]
- 51.Wang T, Satoh F, Morimoto R, Nakamura Y, Sasano H, Auchus RJ, Edwards MA. and Rainey WE. Gene expression profiles in aldosterone-producing adenomas and adjacent adrenal glands. Eur J Endocrinol 2011;164:613-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye P, Mariniello B, Mantero F, Shibata H. and Rainey WE. G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism. J Endocrinol 2007;195:39-48. [DOI] [PubMed] [Google Scholar]
- 53.Davies LA, Hu C, Guagliardo NA, Sen N, Chen X, Talley EM, Carey RM, Bayliss DA. and Barrett PQ. TASK channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci U S A 2008;105:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lenzini L, Caroccia B, Campos AG, Fassina A, Belloni AS, Seccia TM, Kuppusamy M, Ferraro S, Skander G, Bader M, Rainey WE. and Rossi GP. Lower expression of the TWIK-related acid-sensitive K+ channel 2 (TASK-2) gene is a hallmark of aldosterone-producing adenoma causing human primary aldosteronism. J Clin Endocrinol Metab 2014;99:E674-E682. [DOI] [PubMed] [Google Scholar]
- 55.Calhoun DA. and White WB. Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension. J Am Soc Hypertens 2008;2:462-468. [DOI] [PubMed] [Google Scholar]
- 56.Baudrand R, Guarda FJ, Fardella C, Hundemer G, Brown J, Williams G. and Vaidya A. Continuum of Renin-Independent Aldosteronism in Normotension. Hypertension. 2017;69:950-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, Ford I, Cruickshank JK, Caulfield MJ, Salsbury J, Mackenzie I, Padmanabhan S. and Brown MJ. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, Quinkler M, Hanslik G, Lang K, Hahner S, Allolio B, Meisinger C, Holle R, Beuschlein F, Bidlingmaier M. and Endres S. Observational study mortality in treated primary aldosteronism: the German Conn's registry. Hypertension. 2012;60:618-624. [DOI] [PubMed] [Google Scholar]
- 59.Campbell SE, Diaz-Arias AA. and Weber KT. Fibrosis of the human heart and systemic organs in adrenal adenoma. Blood Press 1992;1:149-156. [DOI] [PubMed] [Google Scholar]
- 60.Freel EM, Mark PB, Weir RA, McQuarrie EP, Allan K, Dargie HJ, McClure JD, Jardine AG, Davies E. and Connell JM. Demonstration of blood pressure-independent noninfarct myocardial fibrosis in primary aldosteronism: a cardiac magnetic resonance imaging study. Circ Cardiovasc Imaging. 2012;5:740-747. [DOI] [PubMed] [Google Scholar]
- 61.Napoli C, Di Gregorio F, Leccese M, Abete P, Ambrosio G, Giusti R, Casini A, Ferrara N, De Matteis C, Sibilio G, Donzelli R, Montemarano A, Mazzeo C, Rengo F, Mansi L. and Liguori A. Evidence of exercise-induced myocardial ischemia in patients with primary aldosteronism: the Cross-sectional Primary Aldosteronism and Heart Italian Multicenter Study. J Investig Med 1999;47:212-221. [PubMed] [Google Scholar]
- 62.Brilla CG. Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res 1990;67:1355-1364. [DOI] [PubMed] [Google Scholar]
- 63.Brilla CG, Matsubara LS. and Weber KT. Anti-aldosterone treatment and the prevention of myocardial fibrosis in primary and secondary hyperaldosteronism. J Mol Cell Cardiol 1993;25:563-575. [DOI] [PubMed] [Google Scholar]
- 64.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J. and Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709-717. [DOI] [PubMed] [Google Scholar]
- 65.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J. and Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309-1321. [DOI] [PubMed] [Google Scholar]
- 66.Rizzoni D, Porteri E, Castellano M, Bettoni G, Muiesan ML, Tiberio G, Giulini SM, Rossi G, Bernini G. and Agabiti-Rosei E. Endothelial dysfunction in hypertension is independent from the etiology and from vascular structure. Hypertension. 1998;31:335-341. [DOI] [PubMed] [Google Scholar]
- 67.Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, Rabbia F, Federspil G. and Mulatero P. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab 2006;91:454-459. [DOI] [PubMed] [Google Scholar]
- 68.Monticone S, D'Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F. and Mulatero P. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2018;6:41-50. [DOI] [PubMed] [Google Scholar]
- 69.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, Lloberes P, Diaz de Atauri MJ, Somoza M, Masa JF, Gonzalez M, Sacristan L, Barbe F, Duran-Cantolla J, Aizpuru F, Manas E, Barreiro B, Mosteiro M, Cebrian JJ, de la Pena M, Garcia-Rio F, Maimo A, Zapater J, Hernandez C, Grau SanMarti N. and Montserrat JM. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 2013;310:2407-2415. [DOI] [PubMed] [Google Scholar]
- 70.Calhoun DA, Nishizaka MK, Zaman MA. and Harding SM. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest. 2004;125:112-117. [DOI] [PubMed] [Google Scholar]
- 71.Raizman JE, Diamandis EP, Holmes D, Stowasser M, Auchus R. and Cavalier E. A renin-ssance in primary aldosteronism testing: obstacles and opportunities for screening, diagnosis, and management. Clin Chem 2015;61:1022-1027. [DOI] [PubMed] [Google Scholar]
- 72.Kerstens MN, Kobold AC, Volmer M, Koerts J, Sluiter WJ. and Dullaart RP. Reference values for aldosterone-renin ratios in normotensive individuals and effect of changes in dietary sodium consumption. Clin Chem 2011;57:1607-1611. [DOI] [PubMed] [Google Scholar]
- 73.Hannemann A, Friedrich N, Ludemann J, Volzke H, Rettig R, Peters J, Reincke M, Doring A, Nauck M. and Wallaschofski H. Reference intervals for aldosterone, renin, and the aldosterone-to-renin ratio in the population-based Study of Health in Pomerania (SHIP-1). Horm Metab Res 2010;42:392-399. [DOI] [PubMed] [Google Scholar]
- 74.Nishizaka MK, Pratt-Ubunama M, Zaman MA, Cofield S. and Calhoun DA. Validity of plasma aldosterone-to-renin activity ratio in African American and white subjects with resistant hypertension. Am J Hypertens 2005;18:805-812. [DOI] [PubMed] [Google Scholar]
- 75.Montori VM. Validity of the aldosterone-renin ratio used to screen for primary aldosteronism. Mayo Clin Proc 2001;76:877-882. [DOI] [PubMed] [Google Scholar]
- 76.Rye P, Chin A, Pasieka J, So B, Harvey A. and Kline G. Unadjusted Plasma Renin Activity as a "First-Look" Test to Decide Upon Further Investigations for Primary Aldosteronism. J Clin Hypertens (Greenwich). 2015;17:541-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haase M, Riester A, Kropil P, Hahner S, Degenhart C, Willenberg HS. and Reincke M. Outcome of adrenal vein sampling performed during concurrent mineralocorticoid receptor antagonist therapy. J Clin Endocrinol Metab 2014;99:4397-4402. [DOI] [PubMed] [Google Scholar]
- 78.Baudrand R, Guarda FJ, Torrey J, Williams G. and Vaidya A. Dietary Sodium Restriction Increases the Risk of Misinterpreting Mild Cases of Primary Aldosteronism. J Clin Endocrinol Metab 2016;101:3989-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williams TA, Lenders JWM, Mulatero P, Burrello J, Rottenkolber M, Adolf C, Satoh F, Amar L, Quinkler M, Deinum J, Beuschlein F, Kitamoto KK, Pham U, Morimoto R, Umakoshi H, Prejbisz A, Kocjan T, Naruse M, Stowasser M, Nishikawa T, Young WF, Jr., Gomez-Sanchez CE,Funder JW. and Reincke M. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol 2017;5:689-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sukor N, Gordon RD, Ku YK, Jones M. and Stowasser M. Role of unilateral adrenalectomy in bilateral primary aldosteronism: a 22-year single center experience. J Clin Endocrinol Metab 2009;94:2437-2445. [DOI] [PubMed] [Google Scholar]
- 81.Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, Satoh F. and Young WF., Jr. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63:151-160. [DOI] [PubMed] [Google Scholar]
- 82.Pandey A, Raza F, Velasco A, Brinker S, Ayers C, Das SR, Morisky DE, Halm EA. and Vongpatanasin W. Comparison of Morisky Medication Adherence Scale with therapeutic drug monitoring in apparent treatment-resistant hypertension. J Am Soc Hypertens 2015;9:420-426.e422. [DOI] [PubMed] [Google Scholar]
- 83.Velasco A, Chung O, Raza F, Pandey A, Brinker S, Arbique D, Price A, Lotan Y, Das SR. and Vongpatanasin W. Cost-Effectiveness of Therapeutic Drug Monitoring in Diagnosing Primary Aldosteronism in Patients With Resistant Hypertension. J Clin Hypertens (Greenwich). 2015;17:713-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghose RP, Hall PM. and Bravo EL. Medical management of aldosterone-producing adenomas. Ann Intern Med 1999;131:105-108. [DOI] [PubMed] [Google Scholar]
- 85.Batterink J, Stabler SN, Tejani AM. and Fowkes CT. Spironolactone for hypertension. Cochrane Database Syst Rev 2010;8:CD008169. [DOI] [PubMed] [Google Scholar]
- 86.Hundemer GL, Curhan GC, Yozamp N, Wang M. and Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol 2018;6:51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huffman DH, Kampmann JP, Hignite CE. and Azarnoff DL. Gynecomastia induced in normal males by spironolactone. Clin Pharmacol Ther 1978;24:465-473. [DOI] [PubMed] [Google Scholar]
- 88.Levy DG, Rocha R. and Funder JW. Distinguishing the antihypertensive and electrolyte effects of eplerenone. J Clin Endocrinol Metab 2004;89:2736-2740. [DOI] [PubMed] [Google Scholar]
- 89.Tam TS, Wu MH, Masson SC, Tsang MP, Stabler SN, Kinkade A, Tung A. and Tejani AM. Eplerenone for hypertension. Cochrane Database Syst Rev 2017;2:CD008996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu VC, Chueh SJ, Chen L, Chang CH, Hu YH, Lin YH, Wu KD, Yang WS. and Group TS. Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens 2017;35:1698-1708. [DOI] [PubMed] [Google Scholar]
- 91.Arlt W, Lang K, Sitch AJ, Dietz AS, Rhayem Y, Bancos I, Feuchtinger A, Chortis V, Gilligan LC, Ludwig P, Riester A, Asbach E, Hughes BA, O'Neil DM, Bidlingmaier M, Tomlinson JW, Hassan-Smith ZK, Rees DA, Adolf C, Hahner S, Quinkler M, Dekkers T, Deinum J, Biehl M, Keevil BG, Shackleton CHL, Deeks JJ, Walch AK, Beuschlein F. and Reincke M. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight 2017;2:e93136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sywak M. and Pasieka JL. Long-term follow-up and cost benefit of adrenalectomy in patients with primary hyperaldosteronism. Br J Surg 2002;89:1587-1593. [DOI] [PubMed] [Google Scholar]
- 93.Brown JM, Robinson-Cohen C, Luque-Fernandez MA, Allison MA, Baudrand R, Ix JH, Kestenbaum B, de Boer IH. and Vaidya A. The Spectrum of Subclinical Primary Aldosteronism and Incident Hypertension: A Cohort Study. Ann Intern Med 2017;167:630-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vaclavik J, Sedlak R, Plachy M, Navratil K, Plasek J, Jarkovsky J, Vaclavik T, Husar R, Kocianova E. and Taborsky M. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomized, double-blind, placebo-controlled trial. Hypertension. 2011;57:1069-1075. [DOI] [PubMed] [Google Scholar]
- 95.Lubitz CC, Economopoulos KP, Sy S, Johanson C, Kunzel HE, Reincke M, Gazelle GS, Weinstein MC. and Gaziano TA. Cost-Effectiveness of Screening for Primary Aldosteronism and Subtype Diagnosis in the Resistant Hypertensive Patients. Circ Cardiovasc Qual Outcomes. 2015;8:621-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Satoh F, Morimoto R, Ono Y, Iwakura Y, Omata K, Kudo M, Takase K, Seiji K, Sasamoto H, Honma S, Okuyama M, Yamashita K, Gomez-Sanchez CE, Rainey WE, Arai Y, Sasano H, Nakamura Y. and Ito S. Measurement of peripheral plasma 18-oxocortisol can discriminate unilateral adenoma from bilateral diseases in patients with primary aldosteronism. Hypertension. 2015;65:1096-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eisenhofer G, Dekkers T, Peitzsch M, Dietz AS, Bidlingmaier M, Treitl M, Williams TA, Bornstein SR, Haase M, Rump LC, Willenberg HS, Beuschlein F, Deinum J, Lenders JW. and Reincke M. Mass Spectrometry-Based Adrenal and Peripheral Venous Steroid Profiling for Subtyping Primary Aldosteronism. Clin Chem 2016;62:514-524. [DOI] [PubMed] [Google Scholar]
- 98.Williams TA, Peitzsch M, Dietz AS, Dekkers T, Bidlingmaier M, Riester A, Treitl M, Rhayem Y, Beuschlein F, Lenders JW, Deinum J, Eisenhofer G. and Reincke M. Genotype-Specific Steroid Profiles Associated With Aldosterone-Producing Adenomas. Hypertension. 2016;67:139-145.</References> [DOI] [PubMed] [Google Scholar]