Figure 1.
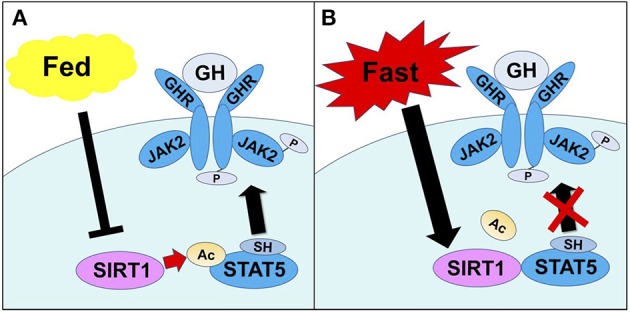
The mechanisms through which SIRT1 regulates STAT5 activation by GH. (A) In the fed condition, the SH2 domain of STAT5 recognizes and binds to Tyr-phosphorylated GHR, causing JAK2 to phosphorylate and activate STAT5. (B) In the fasting condition, SIRT1 is activated and interacts with STAT5, thereby deacetylating Lys residues adjacent to the SH2 domain of STAT5. This results in an impaired ability to bind Tyr-phosphorylated GHR, which inhibits activation of STAT5. Excerpt from, Yamamoto et al. (60).
