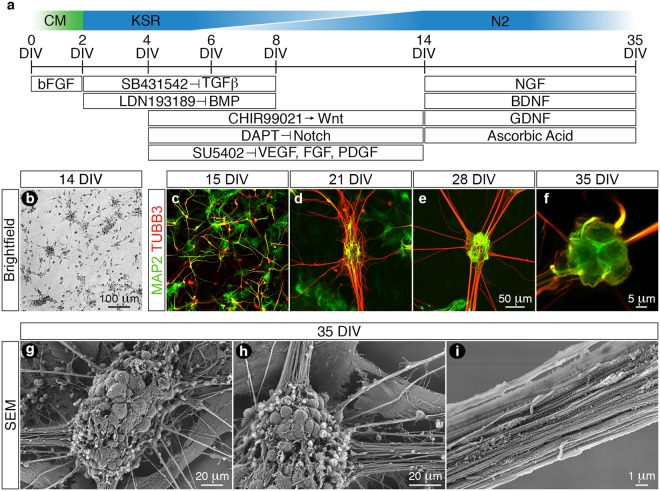
Figure 1.
Differentiation of in vitro peripheral sensory neurons from hESCs. (a) Schematic diagram detailing the differentiation protocol employed in this study. hESCs were seeded as single cells and cultured in MEF conditioned medium until optimal confluence was reached (0–2 DIV). The hESCs were then differentiated using a combination of dual-SMAD inhibition and early WNT activation coupled with small-molecule inhibition of Notch, VEGF, FGF and PDGF signaling pathways (2–14 DIV). (b) Following completion of the differentiation phase (14 DIV) the derived cells exhibited typical immature neuronal morphology with each individual cell elaborating several neurites. (c–f) The cells (14 DIV) were replated in N2 medium supplemented with a defined neurotrophic factor cocktail (BDNF, GDNF, NGF and ascorbic acid) and time-course analyses using the pan-neuronal markers MAP2 and TUBB3 demonstrate that the differentiated neurons become migratory and transform over time from an arborised monolayer (c, 15 DIV) into neuronal ganglia that radially project axonal tracts (f, 35 DIV). (g–i) SEM analysis of the in vitro neuronal ganglia (35 DIV) demonstrates the intimate association of several neuronal somata of varying size classes (g) from which axons project radially away (h) and assemble into fasciculated nerve tracts (i). All images were derived from at least three independent differentiation experiments. Abbreviations: CM, conditioned medium; DIV, days in vitro; SEM, scanning electron microscopy. Scale bars: (b) 100 μm; (c–e) 50 μm; (f) 5 μm; (g–h) 20 μm; (i) 1 μm.