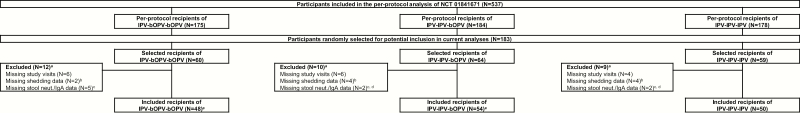
Figure 1.
Flow diagram for the selection of the analytical cohort from NCT 01841671, an investigation of the “Immunogenicity of 1 or 2 Doses of bOPV in Chilean Infants Primed with IPV Vaccine (IPV002ABMG),” undertaken in Santiago, Chile, between 25 April and 1 August 2013.
Abbreviations: bOPV, bivalent oral polio vaccine; IgA, immunoglobulin A; IPV, trivalent inactivated polio vaccine; neut., neutralization.
aReasons for exclusion are not mutually exclusive.
bSubjects with missing type 2 oral poliovirus vaccine shedding data from at least 1 visit at 28, 29, 30, 31, or 32 weeks of age.
cSubjects with missing polio type 2–specific stool neutralization and immunoglobulin A data from at least 1 visit at 28 or 30 weeks of age.
dPolio type 1–specific immunoglobulin A data were missing from the visit at 30 weeks of age in 2 of the included subjects in the IPV-IPV-bOPV group and 1 in the IPV-IPV-IPV group.
eType 1– and 2–specific serum neutralization data were missing from the visits at 28 and/or 29 weeks of age in 1 of the included subjects in the IPV-IPV-bOPV group and 3 in the IPV-bOPV-bOPV group.