Figure 1. FIGURE 1: Current model for the biosynthesis, secretion, uptake and recycling of pyoverdines in P. fluorescens A506.
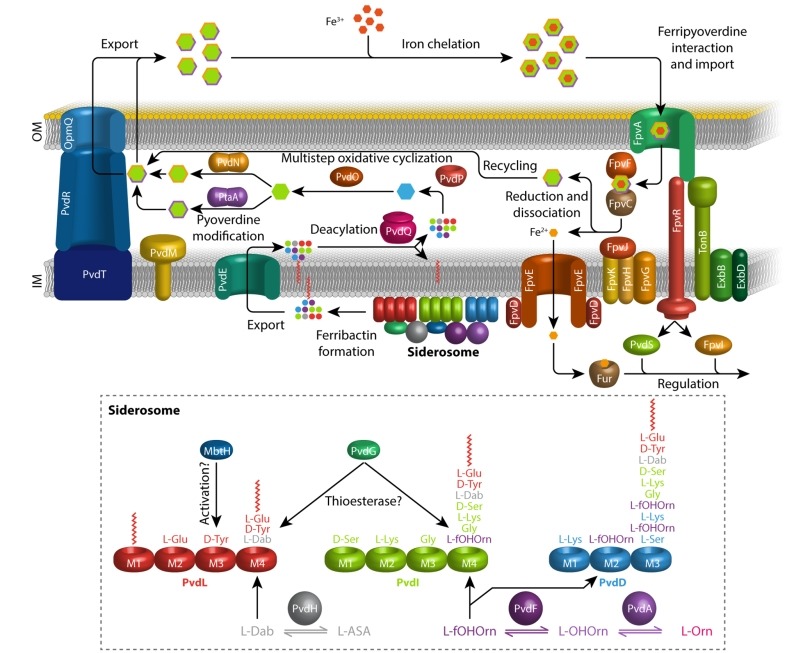
The acylated ferribactin precursor is synthesized in the cytoplasm by NRPSs and auxiliary enzymes organized in membrane associated complexes termed “siderosomes”. The cytoplasmic synthesis is detailed in the box at the bottom. PvdL synthesizes the conserved N-terminal tripeptide with its acylation, the other NRPS are responsible for rest of the peptide and therefore vary between strains with distinct sequences. The auxiliary enzymes MbtH, PvdG, PvdH, PvdA, PvdF, and PvdD play the indicated roles (see text for details). The acylated ferribactin is exported most likely by PvdE into the periplasm, where it is deacylated by the Ntn-type hydrolase PvdQ. Subsequently, PvdP catalyzes the oxidative cyclization, resulting in dihydropyoverdine. PvdO, possibly in conjunction with other proteins, facilitates the final oxidation, yielding the characteristic pyoverdine chromophore. Thereafter, side-chain modification-pathways transform the original L-glutamic acid side chain either to the succinamide, catalyzed by PvdN, or the α-ketoglutarate, catalyzed by PtaA. The modified pyoverdines are then secreted via various transport systems such as PvdRT-OmpQ, and bind outside ferric iron. The complex binds to FpvA and is TonB-dependently taken up. FpvF and FpvC reduce and dechelate the iron, which is taken up by the FpvDE transporter. The apo pyoverdine is recycled. See text for details.
