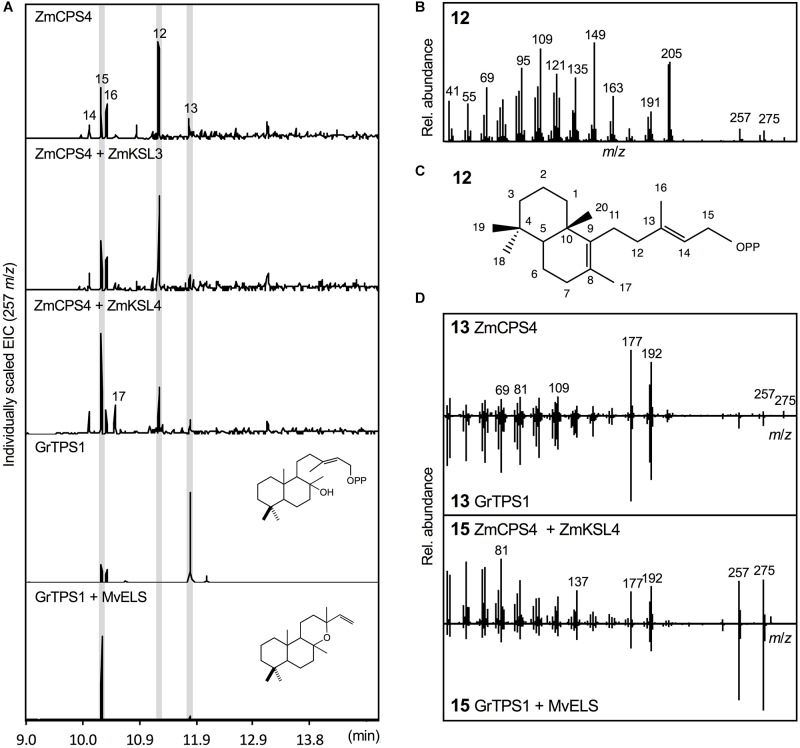
FIGURE 4.
Functional characterization of the maize diterpene synthase ZmCPS4. (A) Extracted ion chromatograms (EIC, m/z 257) of reaction products resulting from E. coli co-expression assays of ZmCPS4 alone or in combination with the ent-kaurene synthase ZmKSL3 (Fu et al., 2016) or the dolabradiene synthase ZmKSL4 (Mafu et al., 2018). Expression of the LPP synthase GrTPS1 (Zerbe et al., 2013) was used to produce a labda-13-en-8-ol (i.e., dephosphorylated labda-13-en-8-ol diphosphate, LPP) standard 13, and co-expression of GrTPS1 and the multi-functional class I diTPS MvELS (Zerbe et al., 2014) enabled the formation of a manoyl oxide 15 standard. (B) Mass spectrum of the ZmCPS4 product, 8,13-copalol (i.e., dephosphorylated 8,13-CPP) 12. (C) Structural identification of the ZmCPS4 product as 8,13-CPP as verified by NMR analysis. Compounds 14, 16, and 17 represent unidentified LPP and 8,13-CPP derivatives. (D) Comparison of mass spectra of the ZmCPS4 products, compounds 13 and 15 to those of authentic standards of labda-13-en-8-ol (i.e., dephosphorylated labda-13-en-8-ol diphosphate, LPP) produced by GrTPS1 and manoyl oxide produced by combining GrTS1 and MvELS. Where applicable, depicted structures represent the native prenyl diphosphate class II diTPS products, whereas spectra are derived from the corresponding dephosphorylated compounds that are formed during enzyme co-expression analyses by the activity of endogenous E. coli phosphatases.