Table 1.
Correlation between Aldehyde Electrophilicity and Enantioselectivity in the anti-(α-Aryl)allylation of Aromatic Aldehydes and Fluoral Hydrate.a
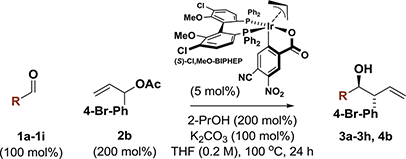 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | σ | 1a-1i | 3a-3h | Yield% | dr | ee% |
| 1 | 4-Me2N-Ph | -0.83 | 1a | 3a | 16 | 4:1 | 1 |
| 2 | 4-MeO-Ph | -0.27 | 1b | 3b | 54 | 9:1 | 5 |
| 3 | 4-Me-Ph | -0.17 | 1c | 3c | 69 | 4:1 | 12 |
| 4 | Ph | 0.00 | 1d | 3d | 74 | 4:1 | 15 |
| 5 | 4-F-Ph | 0.06 | 1e | 3e | 88 | 3:1 | 16 |
| 6 | 4-Br-Ph | 0.23 | 1f | 3f | 88 | 4:1 | 23 |
| 7 | 3-(6-Br-Pyr) | - | 19 | 3g | 82 | 5:1 | 37 |
| 8 | 2-(6-Br-Pyr) | - | 1h | 3h | 75 | 4:1 | 65 |
| 9b | CF3 | - | 1i | 4b | 88 | >20:1 | 94 |
Yields of material isolated by silica gel chromatography. Enantioselectivities were determined by chiral stationary phase HPLC analysis. See Supporting Information for further experimental details.
4Å molecular sieves 300 wt%). Fluoral hydrate in water (75 wt%).
