Abstract
Background and Aim
Drug‐induced autoimmune hepatitis (DIAIH) is an adverse effect associated with several drugs that usually occurs acutely, with variable latency, and it may potentially be mortal. There are a few reports and studies about DIAIH.
Methods
This was an analytical study of a retrospective cohort of patients, discriminated according to idiopathic or drug‐induced etiology, followed up for a 7‐year period until 31 December 2016.
Results
A total of 190 patients were selected for the analysis, 12 (6.3%) with DIAIH. The two main drugs related to DIAIH were nitrofurantoin, n = 8 (67%), and NSAID, n = 2 (17%), constituting 84% of the cases. There were no significant differences in seropositivity between AIH with DIAIH in antinuclear antibodies (ANA) and anti‐smooth muscle antibodies (ASMA) antibodies, with 82.6% versus 82.6% and 34% versus 16%, respectively. The fibrosis stages were similar, except for the F4 stage, in a greater proportion in AIH. None of the patients with DIAIH had cirrhosis or developed it during follow‐up, but it was present in 42.1% of the AIH cases at diagnosis (P = 0.003). Biochemical remission with management was higher in DIAIH but not significant (91.7% vs 80.9%, P = 0.35). The definitive interruption of immunosuppression was successfully performed in 25% of those with DIAIH without relapses but was only possible in 2.8% in AIH (P < 0.001) with 32 cases of relapses.
Conclusion
DIAIH constitutes a minor proportion of AIH. The clinical and histological characteristics may be similar; DIAIH patients have a greater chance of having treatment suspended with a low risk of relapse, progression to cirrhosis, or need for liver transplant.
Keywords: autoimmune hepatitis, autoimmunity, drug‐induced liver injury, immunosuppression, prognosis
Introduction
Drug‐induced autoimmune hepatitis (DIAIH) is a form of idiosyncratic hepatotoxicity characterized by acute or chronic liver injury. This is associated with autoantibodies directed toward proteins expressed in the hepatocytes1 as a consequence of unstable metabolites of drugs that react with cell components, especially proteins of the p450 cytochrome system recognized as neoantigens1, 2 with autoimmune hepatitis (AIH) as a result, with mechanisms cleared up for certain drugs. This type of autoimmunity is characterized by the presence of antinuclear antibodies (ANA), anti‐smooth muscle or the elevation of gamma globulin, and/or the presence of specific haplotypes of human leukocyte antigen (HLA). It differentiates from other forms of hepatotoxicity where autoantibodies are generally negative.
More than 1000 drugs and different herbal products have been described as the cause of drug‐induced liver injury (DILI) with dose‐dependent toxicity or, more frequently, with idiosyncratic toxicity. Among them, antimicrobials (nitrofurantoin and minocycline), interferon, infliximab, and statins may induce hepatocellular damage that mimics autoimmune hepatic lesions, with a profile of positive antibodies and histological findings distinctive of other forms of hepatic injury.3
However, the differential diagnosis between these entities may be especially difficult because of the unpredictability of adverse responses to medicines, significant clinical heterogeneity, indistinguishable histological findings of AIH from DIAIH,3 unpredictable recurrence by histology,4 and variable latency (from 1 week to 12 months) even in cases caused by the same agent. For this reason, it is not rare that DIAIH may not be differentiated correctly, and the defined proportion could be different from those found in previous reports.
There are several possible combinations of DILI and AIH that have been proposed. First, Liu et al., in 2002,1 distinguished two types, and then, Weiler‐Normann and Schramm, in 2011,5 established a classification of DILI and AIH by proposing possible connections with suggested diagnoses and clinical characteristics—first, AIH with DILI: patients with reactivation of a known AIH on introduction of a new drug, often with advanced fibrosis on histology; second, drug‐induced AIH: patients with unrecognized AIH or a predisposition to AIH, in whom AIH is unmasked or induced by DILI with good response to steroids and relapse after withdrawal of immunosuppression with a permanent need for immunosuppression; and third, immune‐mediated DILI: an acute or chronic process depending on the duration of the exposure to the hepatic insult, which resolves or becomes quiescent with drug withdrawal. Finally, there are patients who present with DILI with positive autoantibodies that must be recognized because of its implications that are not yet recognized.6
Retrospective unicentric series7, 8 show slight variation in the proportion of DIAIH and differences in early response against late response with the immunosuppression management. The reports regarding this in Latin America are very scarce and limited to reports of isolated cases, such as a case of anti‐tumor necrosis factor (TNF)9 reported in Medellín, Colombia, and a case of a teenage patient who started treatment with isotretionin for severe acne and was later diagnosed with DIAIH in Lima, Peru.10
In this study, patients who were characterized in a previous AIH study11 are reevaluated. The objective of the current study is to describe the clinical, paraclinical, and evolutionary characteristics in patients diagnosed with autoimmune and drug‐induced hepatitis in an analytical way. The study focuses on the proportion of AIH and DIAH and on differential responses to immunomodulatory management and relapse risk in a retrospective cohort of patients with confirmed diagnosis of AIH.
Methodology
Design and sampling
This is a study of a retrospective, analytical cohort, with convenience sampling, based on patients with AIH diagnosis according to the 10th revision of the International Classification of Diseases (ICD‐10) in the records of the electronic clinical history of Pablo Tobón Uribe's statistical service of the city of Medellin, Colombia, during the period January 2010 to 31 December 2016.
Patients with a complete clinical history and confirmed diagnosis of AIH by biopsy were included from the moment of diagnostic suspicion, through the periodic follow‐up at the discretion of the hepatologist, and until the last ambulatory or inpatient hospital medical record. The reasons for exclusion were: patients younger than 16 years old, overlap syndromes, hepatic failure, score less than 6 with the simplified AIH criteria, and unavailability of a hepatic biopsy.
In the case of DIAIH, only cases considered related to drugs, according to the clinical judgment, were evaluated with the CIOMS/RUCAM scale (International Organizations of Medical Scientists and Roussel Uclaf Causality Assessment Method Scale).12 The cases classified as possible 3–5, probable 6–8, or highly probable >8 were logged into a database.
All the patients included had complete studies of exclusion of etiologies such as viral [anti‐Hepatitis C virus (HCV), Total hepatitis B core antibody (anti‐HBc), surface antigen of the hepatitis B virus (HBsAg), and Hepatitis B surface antibody (anti‐HBs)]; toxic, metabolic (α‐1 antitrypsin, transferrin saturation, ferritin, ceruloplasmin); and other autoimmune liver diseases (ANCA and antimitochondrial antibodies, AMA), particularly those associated with inflammatory bowel disease such as primary sclerosing cholangitis (PSC).
The computerized database was created by the gastrohepatology group, with variables according to standard definitions. Sociodemographic variables were included (age, gender, race) in addition to the clinical ones, such as the presentation manner: asymptomatic alteration of hepatic biochemistry, unspecified symptoms (asthenia, adynamia, hyporexia, and fever), acute hepatitis (jaundice, abdominal pain, fever, and hypertransaminasemia), or hepatic cirrhosis diagnosed clinically or by any imaging technique. The levels of the ANA, the anti‐smooth muscle antibodies (ASMA), and the AMA are also described, as well as levels of Immunoglobulin G (IgG) serum, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, total bilirubin (TB), serum albumin, prothrombin time (PT), and International Normalized Ratio (INR); the changes in these levels after treatment are recorded.
The histological findings were evaluated at diagnosis by two pathologists with experience in liver biopsies who did not have any knowledge of clinical data and patients’ follow‐ups. The findings were catalogued in a standard manner as follows: typical histology of immune hepatitis: interface hepatitis, lymphoplasmacytic infiltration of portal tracts with extension into the lobe, emperypolesis, and rosette formation; histology compatible with AIH: chronic hepatitis with lymphoplasmacytic infiltration without all the characteristics considered typical; and atypical form: signs of another diagnosis, such as steatohepatitis.13
The degree of hepatic fibrosis was evaluated with the METAVIR scale (F0 to F4): stage 0 (F0) no fibrosis, stage 1 (F1) mild fibrosis, stage 2 (F2) moderate fibrosis, stage 3 (F3) severe fibrosis, and stage 4 (F4) cirrhosis. Criteria of simplified diagnoses of the international autoimmune hepatitis group (IAIHG) were included (<6 points: noncompatible, probable if ≥6 points, or definite if ≥7 points),13 or it was calculated if it had not been recorded.
Biochemical remission, incomplete response, treatment failure, relapse, progression to cirrhosis, indication of liver transplantation, and death related to cirrhosis and to any other cause were evaluated using induction and maintenance treatment.
Biochemical remission was defined as the normalization of transaminase and gamma globulin levels. Relapse was defined as a new rise of ALT>3 times the upper limit of normal (ULN) according to the criteria of the IAIHG or an increase in the levels of IgG. Incomplete response included improvement without reaching remission levels. Failure in treatment included clinical, analytical and histological worsening in spite of treatment.
Statistical analysis
Categorical variables are presented as absolute frequencies or percentages, continuous variables as mean and standard deviation according to normal distribution or median, and interquartile range (IQR) for those not normally distributed according to Kolmogorov–Sminrnov test. The differences among the groups were established with the X2 test for categorical variables and the Mann–Whitney U test for differences in medians. Actuarial probabilities were calculated using the Kaplan–Meier method. The data were censored on the date of the event, at the time of orthotopic liver transplantation (OLT), at the time of the last visit, or the time of completing follow‐up period; the patients with OLT were censored as living patients. All the p values were two‐tail calculated, and values <0.05 were considered statistically significant. Calculations were performed with the SPSS statistical package version 20.1 (SPSS Inc., Chicago, USA).
The preparation and final presentation of the article takes into account STROBE recommendations for the reporting of observational studies.14
The study, considered risk‐free research, remained within the framework of international parameters for studies with human beings, such as the Nuremberg code and Colombian regulations based on resolution 8430 of 1993. The protocol was evaluated and approved by the ethics committee and the Committee for Research on Human Beings of Hospital Pablo Tobón Uribe (HPTU). Given its retrospective nature, no informed consent was required.
Results
During the study period, 362 potential patients with a diagnosis of AIH (ICD‐10) were registered, of whom 172 were excluded (Fig. 1); the main reasons for exclusion were: overlap syndromes (autoimmune hepatitis‐primary biliary cholangitis, AIH‐PBC or autoimmune hepatitis‐primary sclerosing cholangitis, AIH‐PSC) and acute liver failure (due to absence of autoantibodies, no rise of immunoglobulin, and/or limitation to carry out a liver biopsy).
Figure 1.
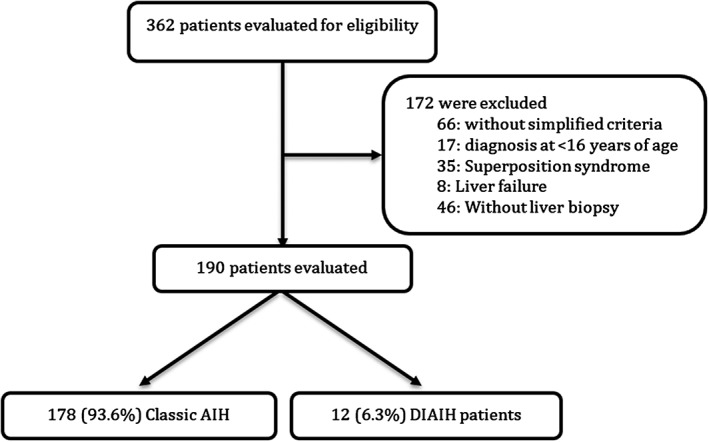
Scheme for the selection of patients. They could have more than one reason to be excluded.
A total of 190 patients were diagnosed with probable or definite AIH according to the simplified criteria; 178 had a diagnosis of AIH and 12 of DIAIH; both groups had similar baseline characteristics, except for the presentation and degree of F4 fibrosis in AIH. The age range was from 16 to 87 years and was similar in both groups, as well as the sex and race patterns where the majority, 92.1% and 91.7%, were females and 96.1% and 91.7% belonged to mixed race in AIH and DIAIH, respectively (Table 1).
Table 1.
Baseline characteristics: comparison of the demographics, seropositivity, AIH score, and histology at presentation in patients with DIAIH and AIH
| Characteristics | AIH (n = 178) | DIAIH (n = 12) | P value |
|---|---|---|---|
| Age at diagnosis – median (IQR)* | 51 (36–59) | 56 (26–56) | 0.40 |
| Gender, female – no. (%) | 164 (92.1) | 11(91.7) | 0.95 |
| Race – no. (%) | |||
| Mixed race | 171 (96.1) | 11 (91.7) | 0.46 |
| Black race | 7 (3.9) | 1 (8.3) | 0.46 |
| History of autoimmunity – no. (%) | 59 (33.1%) | 5(41.7%) | 0.54 |
| Manner of clinical presentation | |||
| Asymptomatic abnormal liver biochemical tests – no. (%) | 35 (19.7) | 0 | 0.80 |
| Unspecified symptoms and abnormal liver biochemical tests – no. (%) | 37 (20.8) | 2 (16.6) | 0.72 |
| Acute hepatitis – no. (%) | 62 (34.8) | 10 (83.3) | <0.001 |
| Liver cirrhosis – no. (%) | 37 (20.8) | 0 (0.0) | 0.07 |
| No data – no. (%) | 7 (3.9) | 0 (0.0) | 0.40 |
| ANA | |||
| Negative – no. (%) | 20 (11.2) | 1 (8.3) | 0.76 |
| 1:40 – no. (%) | 2 (1.1) | 0 (0.0) | |
| ≥1:80 – no. (%) | 145 (81.5) | 11 (91.7) | 0.37 |
| No data – no. (%) | 11 (6.2) | 0 (0.0) | 0.37 |
| ASMA | |||
| Negative – no. (%) | 103 (57.9) | 10 (83.4) | 0.08 |
| 1:40 – no. (%) | 16 (9.0) | 0 (0.0) | 0.27 |
| ≥1:80 – no. (%) | 45 (25.2) | 2 (16.6) | 0.50 |
| No data – no. (%) | 14 (7.9) | 0 (0.0) | 0.31 |
| AMA | |||
| Negative – no. (%) | 159 (89.3) | 12 (100.0) | 0.23 |
| Positive – no. (%) | 10 (5.6) | 0 (0.0) | 0.40 |
| No data – no. (%) | 9 (5.1) | 0 (0.0) | 0.42 |
| Degree of hepatic fibrosis at diagnosis | |||
| F0 – No. (%) | 11 (6.2) | 1 (8.3) | 0.76 |
| F1‐F2 – No. (%) | 35 (19.7) | 4 (33.3) | 0.25 |
| F3‐F4 – No. (%) | 97 (54.4) | 4 (33.3) | 0.15 |
| No data – No. (%)† | 35 (19.7) | 3 (25.0) | 0.65 |
| Histologic finding | |||
| Compatible with AIH – no. (%) | 52 (29.2) | 3 (25.0) | 0.75 |
| Typical of autoimmune hepatitis– no. (%) | 126 (70.8) | 9 (75.0) | 0.75 |
| Simplified score for diagnosis of AIH | |||
| ≥ 7 points (defined AIH) – no. (%) | 104 (58.4) | 8 (66.6) | 0.57 |
| 6 points (probable AIH) – no. (%) | 58 (32.6) | 3 (25.0) | 0.58 |
| < 6 points – no. (%)‡ | 16 (9.0) | 1 (8.3) | 0.58 |
There are no data about state or degree of hepatic fibrosis in the biopsy report.
The diagnosis of these cases was given by AIH criteria and response to treatment.
Mann–Whitney U test was used to establish differences. IQR, interquartile range.
METAVIR: F0, absence of fibrosis; F1, mild fibrosis; F2, moderate fibrosis; F3, severe fibrosis; F4, cirrhosis.
AIH, autoimmune hepatitis; AMA, antimitochondrial antibodies; ANA, antinuclear antibodies; ASMA, anti‐smooth muscle antibodies; DIAIH, drug‐induced autoimmune hepatitis.
The majority of cases (67%) of drug‐induced AIH were attributed to nitrofurantoin, followed by nonsteroidal anti‐inflammatory drugs (NSAIDs) (Fig. 2). None of the patients were exposed to the abovementioned drugs during the follow‐up.
Figure 2.
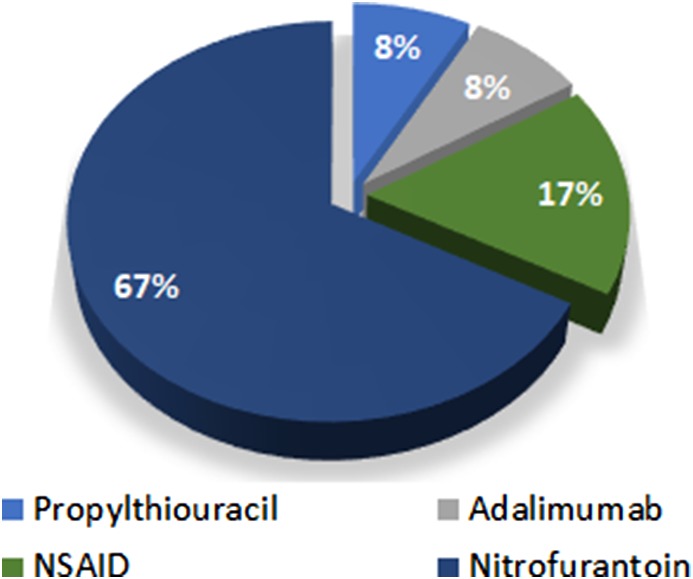
Medicines associated with drug‐induced autoimmune hepatitis.
Acute hepatitis was the main presentation. 34.8% in AIH compared to 83.3% in the presentation was associated with medications (P < 0.001). Liver cirrhosis occurred in one‐fifth of patients with AIH compared to none in those patients with DIAIH (20.3% vs 0%, P = 0.07) (Fig. 3a). No significant differences were observed among the groups by seropositivity profile for the different autoantibodies (Table 1).
Figure 3.
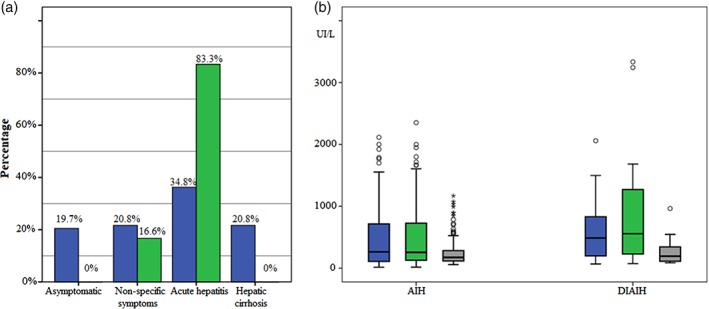
Panel a: Clinical presentation at diagnosis. DIAIH:  , No;
, No;  , Yes. Panel b: comparison of liver biochemical tests at diagnosis of classic hepatitis versus autoimmune hepatitis associated with drugs.
, Yes. Panel b: comparison of liver biochemical tests at diagnosis of classic hepatitis versus autoimmune hepatitis associated with drugs.
 , ALT P = 0.37;
, ALT P = 0.37;  , AST P = 0.37;
, AST P = 0.37;  , ALP P = 0.72.
, ALP P = 0.72.
Patients with DIAIH at the time of diagnosis had higher levels of transaminases, TB, alkaline phosphatase, and PT (Fig. 3b); however, these differences were not statistically significant when compared to values obtained in patients with idiopathic AIH (Table 2).
Table 2.
Comparison of the liver biochemical and function tests at presentation between DIAIH and AIH
| AIH (n = 178) | DIAIH (n = 12) | P value | |||||
|---|---|---|---|---|---|---|---|
| Median | IQR 25–75 | Range | Median | IQR 25–75 | Range | ||
| AST (U/L) | 288 | 133–738 | 15–2352 | 717 | 203–1476 | 69–3334 | 0.37 |
| ALT (U/L) | 272 | 131–700 | 13–2439 | 487 | 192–869 | 69–2059 | 0.37 |
| TB (mg/dL) | 4.3 | 1.0–8.0 | 0.1–41.6 | 13.7 | 0.7–23.3 | 0.5–33 | 0.70 |
| Alkaline phosphatase (U/L) | 186 | 115–285 | 56–1169 | 193 | 111–380 | 84–964 | 0.72 |
| Albumin (mg/dL) | 3.5 | 2.7–4.0 | 1.8–4.8 | 3.3 | 2.5–3.9 | 1.9–4.2 | 0.86 |
| PT (seconds) | 13.1 | 11.4–15.7 | 9.0–26 | 13.4 | 10.8–18.0 | 10–20 | 0.90 |
| INR | 1.2 | 1.0–1.3 | 0.8–2.4 | 1.1 | 1.0–1.5 | 0.8–1.9 | 0.90 |
AIH, autoimmune hepatitis; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DIAIH, drug‐induced autoimmune hepatitis; INR, International Normalized Ratio; IQR, interquartile range; PT, prothrombin time; TB, total bilirubin.
Response to treatment
All patients with DIAIH and 88.8% of the AIH group received induction treatment with steroids and immunomodulators; prednisolone and azathioprine were used the most, with no significant differences between groups. In the maintenance management treatment, there were statistically significant differences; we were able to suspend the steroids in 91.6% patients with DIAIH, compared to 22.5% with AIH (P < 0.001), and we achieved total withdrawal, of any form of immunosuppression (Table 3), in 25% of patients with DIAIH in contrast to 2.8% in those with AIH (P < 0.001). There was a higher biochemical remission in patients with DIAIH, although it was not significant with respect to those with AIH (P = 0.35).
Table 3.
Individual clinical characteristics of patients with drug‐induced AIH
| Gender | Age | Drug | Course | ANA | ASMA | IgG | SCORE AIH | RUCAM Score | ALT | AST | Induction treatment | Maintenance treatment. | Response to therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 22 | NFT | Acute | 1:160 | 1:20 | ND | 6 | 8 | 908 | 440 | AZA, PRED | ND | CR† |
| F | 24 | DIC | Acute | Neg | 1:160 | 3006 | 7 | 4 | 1498 | 3243 | AZA, PRED | AZA | CR‡ |
| F | 78 | NFT | Acute | 1:1280 | Neg | 2629 | 7 | 4 | 354 | 505 | AZA, PRED | AZA | CR‡ |
| F | 66 | AD | Acute | 1:640 | Neg | 2800 | 7 | 5 | 526 | 3334 | AZA, PRED | AZA | CR§ |
| F | 26 | NFT | Acute | 1:160 | 1:20 | 1319 | 7 | 7 | 2059 | 1682 | AZA, PRED | AZA | CR§ |
| F | 28 | PTU | Acute | 1:320 | Neg | ND | 6 | 8 | 754 | 824 | AZA, PRED | AZA | CR§ |
| F | 49 | NFT | Acute | 1:640 | Neg | 4856 | 7 | 4 | 449 | 610 | AZA, PRED | AZA | CR§ |
| F | 62 | DIC IBF |
Chronic | 1:320 | SLA – LP(+) | 1082 | 5 | 4 | 157 | 129 | AZA, PRED | TAC / UCDA | IR§ |
| F | 79 | NFT | Chronic | 1:640 | Neg | 1400 | 5 | 7 | 69 | 73 | AZA, PRED | AZA | CR‡ |
| M | 51 | NFT | Chronic | 1:640 | Neg | 2132 | 7 | 7 | 186 | 279 | AZA, PRED | AZA | CR§ |
| F | 63 | NFT | Chronic | 1:160 | Neg | 2900 | 7 | 7 | 212 | 178 | AZA, PRED | AZA | CR§ |
| F | 87 | NFT | Acute | 1:2560 | 160 | 2000 | 7 | 7 | 650 | 860 | AZA, PRED | AZA | CR§ |
Information not available about discontinuation of immunosuppression.
Discontinuation of immunosuppression successful.
Discontinuation of immunosuppression not tried.
F, female; M, male. Drug: NFT, Nitrofurantoin; DIC, Diclofenac; IBF, Ibuprofen; P, Propylthiouracil; AD, Adalimumab. In patients with SCORE AIH 5, diagnosis was made using adequate response to immunosuppressive treatment.
AIH, autoimmune hepatitis; ALT, alanine aminotransferase; ANA, antinuclear antibodies; ASMA, anti‐smooth muscle antibodies; AST, aspartate aminotransferase; AZA, azathioprine; CR, complete response; IgG, Immunoglobulin G; IC, incomplete response; ND, no data available; PDN, prednisone; TAC, tacrolimus; UCDA, ursodeoxycholic acid.
At an average follow‐up time of 47.4 months (range 1–196) and 43.3 months (range 1–94) of idiopathic AIH and DIAIH, respectively, no patient with DIAIH presented cirrhosis at diagnosis or developed it, relapsed, required liver transplantation, or died during the follow‐up (Table 3); this was different from those patients with AIH where 18% relapsed, 12.5% developed cirrhosis, 5.6% required liver transplantation, and approximately 3% died (Table 4 and Fig. 4a). In addition, the response to the treatment was significantly faster in DIAIH patients (2 months vs 16.8 months, P < 0.001) (Fig. 4b). At the end of follow‐up, no patient in the two groups developed liver failure.
Table 4.
Comparison of treatment and follow‐up: characteristics of patients with AIH versus DIAIH
| Characteristics | AIH (n = 178) | DIAIH (n = 12) | P value |
|---|---|---|---|
| Induction treatment – no. (%) | |||
| None – not indicated – no. (%) | 5 (2.9) | 0 (0.0) | 0.55 |
| Steroids only – no. (%) | 8 (4.6) | 0 (0.0) | 0.45 |
| Steroids and immunomodulator – no. (%) | 158 (90.8) | 12 (100) | 0.15 |
| Immunomodulator only – no. (%) | 3 (1.7) | 0 (0.0) | 0.65 |
| Response to treatment | |||
| Biochemical remission – no. (%) | 144 (80.9) | 11 (91.7) | 0.35 |
| Partial remission – no. (%) | 18 (10.1) | 1 (8.3) | 0.80 |
| No answer – no. (%) | 3 (1.7) | 0 (0.0) | 0.65 |
| No data / N/A – no. (%) | 13 (7.3) | 0 (0.0) | 0.33 |
| Maintenance treatment | |||
| None – no. (%) | 5 (2.8) | 0 (0.0) | 0.55 |
| Steroids only – no. (%) | 9 (5.1) | 0 (0.0) | 0.78 |
| Steroids and immunomodulator – no. (%) | 118 (66.3) | 1 (8.3) | < 0.001 |
| Immunomodulator only – no. (%) | 35 (19.7) | 8 (66.6) | <0.001 |
| Suspension of immunosuppression – no. (%) | 5 (2.8) | 3 (25.0) | <0.001 |
| No data / N/A – no. (%) | 6 (3.3) | 0 (0.0) | 0.50 |
| Relapse – no. (%) | 32 (18.0) | 0 (0.0) | 0.10 |
| Development of cirrhosis during follow‐up – n = 96 † | 12 (12.5) | 0 (0.0) | 0.19 |
| Liver transplantation – n (%) | 10 (5.6) | 0 (0.0) | 0.40 |
| Death during follow‐up – n (%) | 5 (2.8) | 0 (0.0) | 0.55 |
Patients found with cirrhosis at diagnosis were excluded from the calculation.
AIH, autoimmune hepatitis; DIAIH, drug‐induced autoimmune hepatitis.
Figure 4.
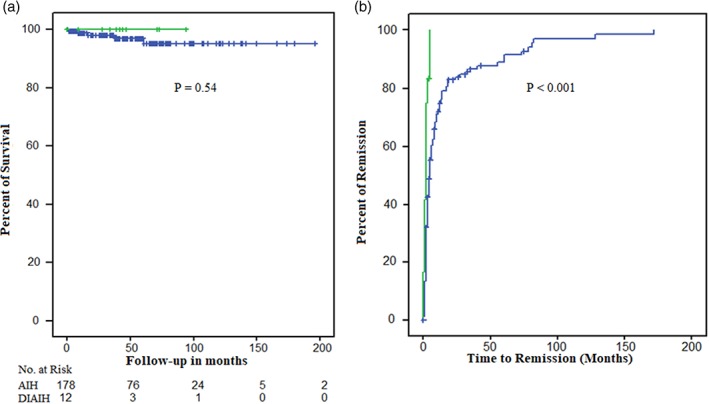
Survival and response to management. Differential survival according to idiopathic or drug‐induced autoimmune hepatitis (panel a) and response to medical management over time (panel b). DIAIH:  , No;
, No;  , Yes.
, Yes.
Discussion
In the present study, the results of a comparative cohort in a reference center demonstrated that, in both groups of patients (AIH and DIAIH), the majority of the population (92%) was female. This is similar to the results reported in series.4, 9, 10 However, unlike these reports, the proportion of DIAIH in this study was somewhat minor; Björnsson et al., in a 10‐year retrospective cohort study from the Mayo Clinic, found 9.2% (24 out of 261)7 and 8.8% (12 out of 136 patients) patients diagnosed with drug hepatotoxicity in an Italian study published by Licata et al.15 This study indicates a proportion of 6.3% of the global AIH patients, confirming variations among countries that are possibly due to population differences and heterogeneous availability in drugs associated with this entity.
Although the relationship between different drugs and AIH has been described, as is evidenced by diverse series and case reports, most cases are limited to a small number of drugs that are extensively marketed, some of them without restriction. In the case of nitrofurantoin and minocycline, the main form of hepatotoxicity is DIAIH in 82% and 73% cases, respectively; methyldopa with less frequency in 55% of cases; and hydralazine in 43% of cases. This was demonstrated in the Drug‐Induced Liver Injury Network (DILIN) prospective study.16 In this study, nitrofurantoin and NSAIDs accounted for 84% of the cases of DIAIH, with nitrofurantoin being 67% higher than that reported in the series described by Björnsson et al.7 and Licata et al.15. However, variability is evident for the NSAIDs, which is not reported in the first case, and in the second case, it is 42% (five cases). In the current study, NSAIDs were involved in 17% of cases of DIAIH. This demonstrates its frequent association, which was previously considered exceptional,17, 18 but still with unclear etiopathogenesis.
In contrast to Björnsson's et al.7 findings, which report an equal number of cases of minocycline with nitrofurantoin in DIAIH,3 and the prospective DILIN study,16 in which minocycline is the second cause of DIAIH, our cohort does not register cases secondary to minocycline given its lower commercialization and difficulty to find it in Colombia despite INVIMA's (National Institute of Drug Vigilance) approval and registration in 2008. This could explain the lower proportion of AIH associated with drugs in Colombia with respect to the studies mentioned.
In relation to anti‐TNF monoclonal antibodies, adalimumab constituted 8% (n = 1) of the cases in this study, contrasting with previous isolated records.9 They have recently begun to constitute an important cause of DIAIH according to the findings of Rodrigues et al. in a series of eight cases (seven with infliximab and one with adalimumab) in a single center in Portugal19 and Björnsson et al. in a cohort in Iceland, where 11 of 15 cases were secondary to infliximab.20 In both series, there was an early response (earlier than 2 months) to the withdrawal of the drug, along with short‐term immunosuppression without documented relapses. This is similar to this study's findings of early transaminase normalization (7 weeks) and no relapse during the 28‐month follow‐up.
While propylthiouracil (PTU) is still a commonly used drug in the treatment of hyperthyroidism, symptomatic hepatic injury due to PTU is not uncommon, and it usually develops within the first month of exposure; however, it is generally benign and nonautoimmune, and it does not justify withdrawing the drug as is explained by the longer series described by Lian et al.21 The case of a young woman (28 years old) with a history of Graves’ disease and acute presentation of symptoms consistent with hepatitis and marked jaundice is reported. As in this case, PTU‐induced AIH is reported in the female population with a history of Graves’ disease.22 Unlike other forms of hepatotoxicity by PTU, this context forces the definitive discontinuation of the drug due to recurrence of the disease with reexposure23 due to the fact that there are even reports of liver failure with prolonged exposure.24
As in the studies, series, and case reports cited, we did not report progression to cirrhosis in any patient with DIAIH during the follow‐up. No patient presented relapse or required transplantation, and because the associated medication was suspended in all of them, no cases of hepatic failure were reported, which has been reported in the setting of AIH.24 In addition, it was possible to discontinue immunosuppression, suggesting that these patients have a better prognosis than patients with idiopathic AIH.
Regarding the histological characteristics as initially demonstrated by Björnsson et al.7 and later by Suzuki et al.3, the histological characteristics do not allow distinction between one or the other entity, except the higher degree of fibrosis in AIH than in DIAIH.3, 7 However, a new diagnostic scale to facilitate the diagnosis of DILI in Japan, the Digestive Disease Week Japan 2004 (DDW‐J) scale, which is highly sensitive and specific, was developed by modifying the Council for International Organizations of Medical Sciences/the Roussel Uclaf Causality Assessment Method (CIOMS/RUCAM) scale.25 Recently, Akemi Tsutsui et al.26 demonstrated that the DDW‐J scale was useful for differentiating AIH from DILI in cases with a DDW‐J scale score of ≥5 in 20 patients in whom it was difficult to differentiate autoimmune liver disease from DILI. The histological features of AIH were characterized by cobblestone hepatocellular change, higher interface hepatitis, and plasma cell infiltration of the portal region. But this diagnostic scale is not well known yet, and it has not been validated in large series, nor was it applied to our patients.
Although in clinical practice, the differentiation between DIAIH and AIH may be challenging because there are no pathognomonic features of AIH, and the diagnosis is usually made according to a clinical setting; we could identify our cases as DIAIH or immune‐mediated DILI according to the classification established by Weiler‐Normann and Schramm because no patient had known AIH, or possibly, some of them had unrecognized or a predisposition to AIH; in addition, there was also good response to steroids, no advanced fibrosis cases, and remission was maintained after withdrawal of steroids.5
Limitations
Among the limitations, apart from being a unicentric study with convenience sampling at a referral hospital, where patients with a spectrum of severe disease are usually referred, which could induce reference bias, the other limitation is the retrospective nature of the study with a limited number of patients in the DIAIH group, which limits the detection of significant differences. Multicenter randomized clinical studies could overcome this limitation and detect differences in progression, remission, and relapse, indications for liver transplantation or death at follow‐up.
During follow‐up, serial paraclinical tests were performed, in which the levels of transaminases, bilirubin, and albumin, among others, were determined. They were performed according to medical criteria. However, the changes in the autoimmunity profile were not reevaluated, and its relationship to the response or the differential profile of HLA with its prognostic implications was also not evaluated.
In addition, the findings of this study, in relation to DIAIH, may possibly be applied only to the type of drugs found (nitrofurantoin, NSAIDs, propylthiouracil, and the anti‐TNF Adalimumab) so that differences in severity and prognosis might be found for different drugs with known association.
One of the study's strengths is the fact that it is the first study in Latin America with well‐characterized sampling, periodic clinical follow‐up, evaluation of response to treatment, and population representativeness, even though the patients are from different regions of Colombia.
In conclusion, DIAIH is an important form of hepatotoxicity; although it is not so frequent in cases of AIH. It should be recognized as these patients have a favorable clinical course and prognosis when suspending the drugs involved; in all the cases, the definitive discontinuation of immunosuppressive drugs must be attempted due to the low risk of relapse, progression to cirrhosis, or need for transplantation as is evidenced in this and previous series of studies.
Declaration of conflict of interest: None declared given the exploratory and retrospective nature of the study, the lack of data in the region and in Latin America, and the financing of our own resources.
References
- 1. Liu Z, Kaplowitz N. Immune‐mediated drug‐induced liver disease. Clin. Liver Dis. 2002; 6: 755–74. [DOI] [PubMed] [Google Scholar]
- 2. Obermayer‐straub P, Strassburg CP, Manns MP, Strassburg CP, Target MPM. Target proteins in human autoimmunity: cytochromes glucuronosyltransferases. Can. J. Gastroenterol. 2000; 14: 429–39. [DOI] [PubMed] [Google Scholar]
- 3. Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug‐induced liver injury. Hepatology. 2011; 54: 931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hisamochi A, Kage M, Ide T et al An analysis of drug‐induced liver injury, which showed histological findings similar to autoimmune hepatitis. J. Gastroenterol. 2016; 51: 597–607. [DOI] [PubMed] [Google Scholar]
- 5. Weiler‐Normann C, Schramm C. Drug induced liver injury and its relationship to autoimmune hepatitis. J Hepatol. 2011; 55: 747–9. [DOI] [PubMed] [Google Scholar]
- 6. Castiella A, Zapata E, Lucena MI, Andrade RJ. Drug‐induced autoimmune liver disease: a diagnostic dilemma of an increasingly reported disease. World J Hepatol. 2014; 6: 160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. E1 B, Talwalkar J, Treeprasertsuk S et al Drug‐induced autoimmune hepatitis: clinical characteristics and prognosis. Hepatology. 2010; 51: 2040–8. [DOI] [PubMed] [Google Scholar]
- 8. Heurgué A, Bernard‐Chabert B, Diebold M, Vitry F, Louvet HGP. Drug‐induced autoimmune hepatitis: a frequent disorder. Gut. 2007; 56 (Suppl. III): A271. [Google Scholar]
- 9. Aristizábal N, Velásquez CJ, Pinto LF. Hepatitis autoinmune asociada al uso de adalimumab en un paciente con artritis reumatoide. Acta Médica Colomb. 2012; 37: 147–51. [Google Scholar]
- 10. Guzman Rojas P, Gallegos Lopez R, Ciliotta Chehade A, Scavino Y, Morales A, Tagle M. Autoimmune hepatitis induced by isotretionine. Rev Gastroenterol Perú. 2016; 36: 86–9. [PubMed] [Google Scholar]
- 11. Díaz‐Ramírez GS, Marín‐Zuluaga JI, Donado‐Gómez JH, Muñoz‐Maya O, Santos‐Sánchez Ó, Restrepo‐Gutiérrez JC. Characterization of patients with autoimmune hepatitis at an university hospital in Medellín‐Colombia: cohort study. Gastroenterol Hepatol. 2018; 41: 87–96. [DOI] [PubMed] [Google Scholar]
- 12. Day P, Serrano J, Hoofnagle JH. Standardization of nomenclature and causality assessment in drug‐induced liver injury: summary of a clinical research workshop. Hepatology. 2010; 52: 730–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dalekos GN, Krawitt EL, Hennes EM et al Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008; 48: 169–76. [DOI] [PubMed] [Google Scholar]
- 14. Centre C, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007; 335: 20–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Licata A, Maida M, Cabibi D et al Clinical features and outcomes of patients with drug‐induced autoimmune hepatitis: a retrospective cohort study. Dig Liver Dis. 2014; 46: 1116–20. [DOI] [PubMed] [Google Scholar]
- 16. De Boer YS, Kosinski AS, Urban TJ et al Features of autoimmune hepatitis in patients with drug‐induced liver injury. Clin. Gastroenterol Hepatol. 2016; 15: 103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martínez‐Odriozola P, Gutiérrez‐Macías A, Ibarmia‐Lahuerta JM‐SJ. Meloxicam as a cause of drug‐induced autoimmune hepatitis. Dig. Dis. Sci. 2010; 55: 1191–2. [DOI] [PubMed] [Google Scholar]
- 18. Scully LJ, Clarke DBR. Diclofenac induced hepatitis. 3 cases with features of autoimmune chronic active hepatitis. Dig. Dis. Sci. 1993; 38: 744–5. [DOI] [PubMed] [Google Scholar]
- 19. Rodrigues S, Lopes S, Magro F et al Autoimmune hepatitis and anti‐tumor necrosis factor alpha therapy: a single center report of 8 cases. World J. Gastroenterol. 2015; 21: 7584–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Björnsson E, Bergmann O, Jonasson JG, Grondal G, Gudbjornsson B, Olafsson S. Drug‐induced autoimmune hepatitis: response to corticosteroids and lack of relapse after cessation of steroids. Clin Gastroenterol Hepatol. 2017; 15: 1635–6. 10.1016/j.cgh.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 21. Lian XL, Bai Y, Dai WX, Guo ZS, Li WLL. Propylthiouracil‐induced overt hepatic injury in patients with hyperthyroidism. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004; 26: 172–7. [PubMed] [Google Scholar]
- 22. Cui B, Abe M, Hidata S et al Autoimmune hepatitis associated with Graves’ disease. Intern. Med. 2003; 42: 331–5. [DOI] [PubMed] [Google Scholar]
- 23. Sato I, Tsunekawa T, Shinohara Y, Nishio Y, Shimizu Y, Suzuki YYS. A case of autoimmune hepatitis with Graves’ disease treated by propylthiouracil. Nagoya J. Med. Sci. 2011; 73: 205–9. [PMC free article] [PubMed] [Google Scholar]
- 24. Web S, Su M, Posselt A et al Propylthiouracil‐associated liver failure presenting as probable autoimmune hepatitis in a child with Graves’ disease. Pediatr. Transplant. 2006; 10: 525–8. [DOI] [PubMed] [Google Scholar]
- 25. Hanatani T, Sai K, Tohkin M et al A detection algorithm for drug‐induced liver injury in medicalinformation databases using the Japanese diagnostic scale and itscomparison with the Council for International Organizations of MedicalSciences/the Roussel Uclaf Causality Assessment Method sca. Pharmacoepidemiol. Drug Saf. 2014; 23: 984–8. [DOI] [PubMed] [Google Scholar]
- 26. Tsutsui A, Harada K, Tsuneyama K et al Clinicopathological study of autoimmune hepatitis cases that were difficult to differentiate from drug‐induced liver injury. Dig. Dis. 2017; 35: 506–14. [DOI] [PubMed] [Google Scholar]


