Fig. 5: CBL0137 cytotoxicity in TH-MYCN+/+ tumors is associated with inhibition of the FACT/MYCN positive feedback loop.
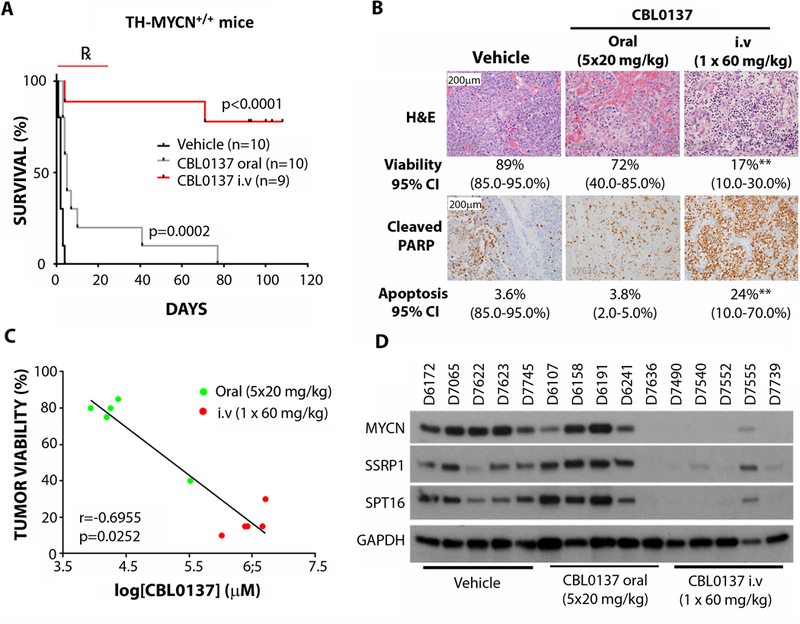
(A) Kaplan Meier plot for survival of 6 week old tumor-bearing TH-MYCN+/+ mice treated with vehicle (5% dextrose given intravenously), oral CBL0137 (20 mg/kg, 5 days/week for 4 weeks) or intravenous CBL0137 (60 mg/kg, once every 4 days for 4 weeks). Log-rank test was used to determine statistical significance between both CBL0137-treated groups, compared to vehicle. Mice began treatment when medium sized (~5 mm diameter) palpable tumors were detected. Endpoint was when tumors reached 10 mm by palpation. ℞ indicates treatment period. (B) Representative images for H&E staining or cleaved PARP (cPARP) in TH-MYCN+/+ tumors treated with oral CBL0137 (5 days at 20 mg/kg/day) or CBL0137 i.v. (1day at 60 mg/kg/day). Quantitation of tumor viability or cPARP positive cells and 95% confidence interval (CI) is displayed for each treatment group. Statistical significance was determined using Mann-Whitney test. **; p<0.01. See table S7 for individual p-values. (C) Correlation of CBL0137 tissue concentration and tumor viability in CBL0137-treated tumors treated as in (B). P-value and R-value determined using Spearman’s rank correlation coefficient. (D) Western blot for MYCN, SSRP1, and SPT16 protein expression in individual TH-MYCN+/+ tumors treated as in (B). GAPDH was used as a loading control. Tumor identifiers are indicated above each lane.
